Metabolic Acidosis
QUICK LOOK AT THE CHAPTER AHEAD
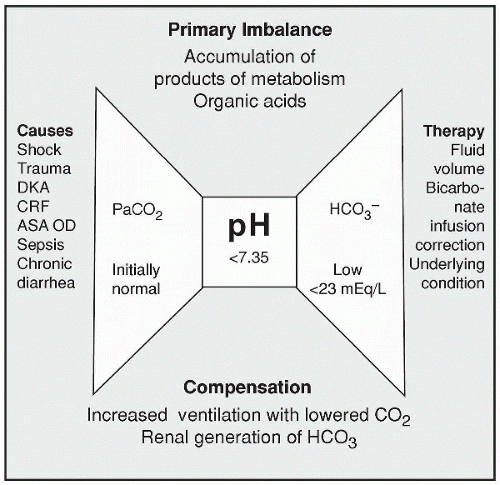
Metabolic acidosis results from a deficiency in HCO3 (< 23 mEq/L) or an excess of noncarbonic acids. In metabolic acidosis the pH falls below 7.35. In this chapter we discuss the causes of metabolic acidosis and the manifestations of the condition and treatment.
Metabolic acidosis is the result of a deficiency in HCO3 (< 23 mEq/L) or of an excess of noncarbonic acids. The pH is below 7.35 in metabolic acidosis.
Bicarbonate Deficit
A bicarbonate deficit can occur through the loss of excessive intestinal secretions such as diarrhea, suction, or fistulas. A loss of HCO3 can also occur through the renal system. Such conditions have the potential to cause a metabolic acidosis.
Excess Acid
Metabolic acidosis that results from increased acid levels can occur in situations involving an accumulation of lactic acid as in shock or cardiac
arrest when anaerobic metabolism occurs due to insufficient oxygenation. Ketoacidosis can also result in metabolic acidosis, particularly with uncontrolled diabetes mellitus but also with excessive alcohol consumption, starvation, or ketogenic weight loss diets. Ingestion of methanol, ethylene glycol, or acetylsalicylic acid can produce extreme situations of metabolic acidosis and possible death. Decreased renal function also leads to a metabolic acidosis due to an inability of the kidneys to secrete H+ into the urine or to conserve HCO3–. This frequently occurs in the elderly as renal function decreases and the kidneys cannot eliminate an increased amount of acid that has accumulated or been ingested.
arrest when anaerobic metabolism occurs due to insufficient oxygenation. Ketoacidosis can also result in metabolic acidosis, particularly with uncontrolled diabetes mellitus but also with excessive alcohol consumption, starvation, or ketogenic weight loss diets. Ingestion of methanol, ethylene glycol, or acetylsalicylic acid can produce extreme situations of metabolic acidosis and possible death. Decreased renal function also leads to a metabolic acidosis due to an inability of the kidneys to secrete H+ into the urine or to conserve HCO3–. This frequently occurs in the elderly as renal function decreases and the kidneys cannot eliminate an increased amount of acid that has accumulated or been ingested.
 Uncontrolled diabetes mellitus, excessive alcohol consumption, starvation, or ketogenic weight loss diets can result in ketoacidosis, which contributes to metabolic acidosis.
Uncontrolled diabetes mellitus, excessive alcohol consumption, starvation, or ketogenic weight loss diets can result in ketoacidosis, which contributes to metabolic acidosis.Hyperchloremic Acidosis
Hyperchloremic acidosis occurs with overtreatment of chloride-type medications, intravenous and hyperalimentation solutions, or increased absorption by the kidneys. Because HCO3– and Cl– are both anions, the HCO3– concentration decreases when an excess of Cl– are available. Ammonium chloride breaks down into NH4+ and Cl–, allowing the ammonium ion to be converted to urea in the liver. This frees the Cl–, allowing it to bind with available H+. The combination forms hydrochloric acid, resulting in a situation of bicarbonate deficit and increased acid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree