CHAPTER 12
Mesenteric Ischemia
Cynthia Christensen
OBJECTIVES
1. Describe the anatomy and physiology relevant to mesenteric ischemia.
2. List the appropriate diagnostic tests relevant to mesenteric ischemia.
3. Discuss the appropriate medical and surgical interventions relevant to mesenteric ischemia.
4. Identify the nursing implications relevant to mesenteric ischemia.
Introduction/Overview
Mesenteric ischemia ensues when blood flow to the intestines ceases or falls below a critical level, resulting in damage to the bowel ranging from completely reversible functional alterations to total hemorrhagic necrosis. The incidence of mesenteric ischemia accounts for 1 in every 1,000 hospital admissions or 9 Americans in every 100,000 (Assar & Zarins, 2008; Broder, 2007; Herbert & Steele, 2007). Mortality rates for mesenteric ischemia range from 30% to 90% depending on the etiology. This has changed little over the past decades despite advances in medical knowledge and technology (Herbert & Steele, 2007). Poor outcomes of mesenteric ischemia result from the delaying in initiating treatment because of the difficulty in arriving at an accurate diagnosis (Assar & Zarins, 2008; Paral et al., 2007). The natural history of mesenteric ischemia includes three possible results:
1. Establishment of competent collateral circulation that will persist effectively throughout the patient’s life or will subsequently break down due to aggravation of the causative factor.
2. Intestinal obstructions without infarction, with blood supply sufficient to sustain life of the gut but not function.
3. Intestinal infarction with the extent of injury varying from a moderate lesion to total necrosis of intestinal tissues (Boley, Brandt, & Sammartano, 1997).
 I. Anatomy: Three arteries with a parallel venous system perfuse most of the gastrointestinal tract. These vessels include the celiac axis, the superior mesenteric artery (SMA), and the inferior mesenteric artery (IMA) (see Fig. 12-1).
I. Anatomy: Three arteries with a parallel venous system perfuse most of the gastrointestinal tract. These vessels include the celiac axis, the superior mesenteric artery (SMA), and the inferior mesenteric artery (IMA) (see Fig. 12-1).
A. The Celiac Axis
1. Originates from the ventral abdominal aorta at the level of T12 or L1
2. Is only about 1.5 cm long
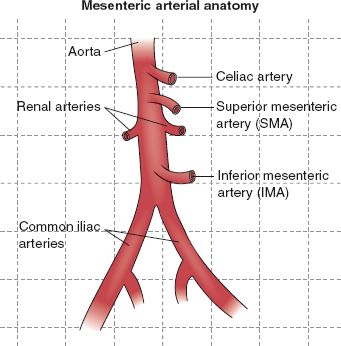
FIGURE 12.1 Mesenteric arterial anatomy.
3. Rapidly divides into the following:
a. Common hepatic
b. Left gastric
c. Splenic arteries
4. The celiac artery provides blood supply to the following:
a. Liver
b. Spleen
c. Pancreas
d. Gastrointestinal tract from the distal esophagus to the second portion of the duodenum
B. The SMA
1. Exits the aorta anteriorly, about 1 to 2 cm below the celiac axis
2. Is the largest and most important intestinal artery
3. Supplies the following:
a. Latter portion of the duodenum
b. Head of pancreas
c. Jejunum
d. Ileum
e. Large bowel proximal to the splenic flexure
C. The IMA
1. Is the smallest of the three intestinal arteries, perfusing only about 10% of the intestine
2. Arises from the aorta about 6 cm distal to the SMA
3. Supplies the left colon and most of the rectum
D. The Venous Anatomy parallels the arterial, eventually draining into the portal system perfusing the liver
1. The superior and inferior mesenteric veins drain the midgut and hindgut
2. The inferior mesenteric vein empties into the splenic vein: these join with the superior mesenteric vein forming the portal vein (Cappell, 1998a; McCutcheon, 2009; Ruotolo & Evans, 1999; Tendler & LaMont, 2011a)
 II. Pathophysiology
II. Pathophysiology
A. Mesenteric Vessels are uniquely reactive to several intrinsic and extrinsic factors affecting blood flow.
1. Blood flow is increased throughout the visceral vasculature in response to a meal. The SMA undergoes a period of hyperemia 20 to 30 minutes after the ingestion of food. The splenic blood flow can demand 10% to 35% of the total cardiac output during this time.
2. The mucosa uses an autoregulatory mechanism to extract increasing amounts of oxygen to preserve its viability during hypoperfusion or metabolic insult.
3. The mesenteric circulation is under the extrinsic control of the nervous and endocrine systems (Herbert & Steele, 2007; Ruotolo & Evans, 1999; Tendler & LaMont, 2011a).
B. The Results of Vascular Insufficiency are not etiology dependent but are determined by multiple factors. Both ischemic and reperfusion syndromes play a role in tissue breakdown and necrosis.
1. Ischemia attacks the most vulnerable area of the bowel first, the mucosa.
a. Mucosal sloughing, ulceration, and hemorrhage occur within 3 hours of ischemic insult.
b. Depending on partial or total occlusion, transmural infarction occurs within 6 hours to several days of ischemic insult.
c. Once intestinal mucosa begins to slough, the underlying tissue becomes susceptible to the contents of the gut and bacteria begins to flourish, allowing the translocation of bacteria and toxins into the systemic circulation resulting in
1) Increased capillary permeability
2) Edema formation
3) Multiorgan dysfunction or failure
2. Reperfusion leads to the production of oxygen-free radicals, which cause disruption of the cell membrane and mucosal barrier. This release of endotoxins can be associated with:
a. Disseminated intravascular coagulation
b. Adult respiratory distress syndrome
c. Sudden cardiovascular collapse (Hirsch et al., 2006; Ruotolo & Evans, 1999)
 III. Etiology/Precipitating Factors. Mesenteric ischemia can be divided into three categories.
III. Etiology/Precipitating Factors. Mesenteric ischemia can be divided into three categories.
A. Acute Mesenteric Ischemia (AMI) occurs when arterial blood and oxygen delivery to the gut is interrupted by the following:
1. Arterial embolism or thrombosis
a. Atherosclerosis
b. Advanced age
c. Low cardiac output states
d. Severe cardiac valvular disease
e. Intra-abdominal malignancy
f. Dysrhythmias
g. Atrial fibrillation
h. Myocardial infarction (Assar & Zarins, 2008; Broder, 2007; Levy, 2007; Tendler & LaMont, 2011a)
2. Mechanical occlusion
a. Trauma
b. Adhesive bands
c. Presence of diagnostic devices within the vessels (Mansour, 1999; Vincente & Kazmers, 1999)
3. Nonocclusive mesenteric ischemia (NOMI) in response to systemic hypoperfusion
a. Congestive heart failure
b. Sepsis
c. Renal failure
d. Hypoperfusion
e. Hemorrhagic shock
f. Hypovolemia
g. Cardiac surgery
h. Medications
1) Digitalis
2) Ergotamines
3) Cocaine
4) Norepinephrine
5) Diuretics
6) Alpha-adrenergic agonists
7) Arginine-vasopressin & angiotensin (Assar & Zarins, 2008; Broder, 2007; Herbert & Steele, 2007; Hirsch et al., 2006; Levy, 2007; Tendler & LaMont, 2011a)
B. Mesenteric Venous Thrombosis (MVT) is the least common cause of AMI. MVT may be primary or idiopathic, or secondary due to one or more other disorders (Assar & Zarins, 2008) such as:
1. Intra-abdominal trauma
2. Sepsis
3. Portal hypertension
4. Hypercoagulable states
a. Protein C deficiency
b. Protein S deficiency
c. Factor V Leiden mutation
d. Antithrombin III deficiency
e. Anticardiolipin antibodies
f. These may be associated with oral contraceptive use
5. Previous abdominal operation
6. Intra-abdominal malignancy
7. Sickle cell disease
8. Smoking
9. Homocysteinemia
10. Immobilization
11. Use of steroids
12. Irritable bowel syndrome
13. Cirrhosis
14. Myeloproliferative syndromes
15. Polycythemia vera
16. Antiphospholipid syndrome
17. Nocturnal paroxysmal hemoglobinuria (Herbert & Steele, 2007; Hugon, deClercq, & Landen, 2007; Levy, 2007; McCutcheon, 2009; Tendler & LaMont, 2011a)
C. Chronic Mesenteric Ischemia (CMI) occurs in the presence of advanced atherosclerosis with insufficient splenic blood flow during periods of increased intestinal demand. Factors associated with this include the following:
1. Atherosclerosis
2. Radiation therapy
3. Celiac compression syndrome
4. Vasculitides
a. Polyarteritis nodosa
b. Takayasu disease
5. Fibromuscular dysplasia
6. Peripheral vascular disease with
a. Advanced age
b. Hypertension
c. Smoking
7. Median arcuate ligament (MAL) syndrome (Gupta, Horan, Turaga, Miller, & Pipinos, 2010; Herbert & Steele, 2007; Kougias, El Sayed, Zhou, & Lin, 2007; Levy, 2007; Tendler & LaMont, 2011b)
 IV. Assessment
IV. Assessment
A. Risk Factors and Primary Prevention
1. Risk factors
a. Cardiac arrhythmias
b. Advanced age
c. Low cardiac output states
d. Generalized atherosclerosis
e. Hypercoagulable states
f. Severe valvular cardiac disease
g. Recent myocardial infarction
h. Intra-abdominal malignancy (Hirsch et al., 2006; McCutcheon, 2009; Tendler & LaMont, 2011a)
2. Primary prevention includes eliminating or controlling those risk factors associated with vascular disease:
a. Hypertension
b. Smoking
c. Hyperlipidemia
d. Diabetes (Nunnelee, 1999)
B. Patient History
1. Subjective findings
a. AMI
1) Rapid onset of peri-umbilical pain out of proportion to examination is the hallmark sign
2) Nausea and vomiting
3) Forceful bowel evacuation
4) Mental status change
5) Feculent odor to breath (Assar & Zarins, 2008; Broder, 2007; Hirsch et al., 2006; Levy, 2007; Tendler & LaMont, 2011a)
b. NOMI
1) Abdominal pain absent in up to 25% of patients
2) Pain varies in character, intensity, and location (Assar & Zarins, 2008; Tendler & LaMont, 2011a)
c. MVT
1) Depends on size of thrombus, size of vessel involved, depth of bowel wall ischemia
2) Diffuse, nonlocalized, moderately severe, constant, colicky type pain disproportionate to examination is the hallmark sign
3) Diarrhea
4) Gastrointestinal bleeding
5) Nausea/vomiting
6) Chills
7) Esophageal variceal hemorrhage
8) Mild abdominal pain evolving over 7 to 10 days
9) May be asymptomatic (Hugon et al., 2007; Levy, 2007; McCutcheon, 2009; Safieddine, Mamazza, Common, & Prabhudesai, 2007)
d. CMI
1) Pain described as “abdominal angina” occurs within 30 minutes of eating, increases in severity, and slowly resolves after 1 to 3 hours
2) Pain may be acute episodes of dull, crampy pain within 1 hour of eating
3) Pain may vary in intensity and radiate to back
4) Diarrhea
5) Nausea/vomiting
6) Constipation
7) Food fear related to pain
8) Early satiety
9) May be asymptomatic (Dupee, 2011; Gupta et al., 2010; Herbert & Steele, 2007; Kougias et al., 2007; Levy, 2007; Tendler & LaMont, 2011b)
2. Objective findings
a. Cachectic appearance
b. Leukocytosis
c. Hypotension
d. Acidosis
e. Pyrexia
f. Increased serum amylase
g. Increased lactate level
h. Hypovolemia
i. Cardiac arrhythmia
j. Weight loss (Broder, 2007; Dupee, 2011; Gupta et al., 2010; Herbert & Steele, 2007; Kougias et al., 2007; Levy, 2007; McCutcheon, 2009; Tendler & LaMont, 2011a, 2011b)
C. Physical Examination
1. Inspection
a. Overall appearance of being uncomfortable
b. Fecal occult blood
c. Abdominal distension
2. Palpation
a. Abdominal pain
1) See subjective finding for description
2) Abdomen soft, nontender with CMI
b. Abdominal rigidity
c. Rebound abdominal tenderness
d. Guarding (except with arterial embolism)
3. Percussion for distension and ascites
4. Auscultation
a. Abdominal or epigastric bruit for CMI
b. Bowel sounds may be absent in AMI
c. Presence of dysrhythmia in NOMI (Assar & Zarins, 2008; Broder, 2007; Herbert & Steele, 2007; Hugon et al., 2007; Levy, 2007; McCutcheon, 2009; Safieddine et al., 2007; Tendler & LaMont, 2011a, 2011b)
D. Considerations Across the Life Span
1. Younger patients develop ischemia but usually have an underlying disease or condition that predisposes them to an abnormality in splanchnic blood flow such as
a. Collagen vascular disease
b. Vasculitis
c. Hypercoagulable states
d. Vasoactive medication
e. Cocaine use
f. Extrinsic compression of the celiac artery
2. Elderly—Mesenteric ischemia is described as a disease of the elderly with most occurring within the sixth or seventh decade of life (Assar & Zarins, 2008; Broder, 2007; Herbert & Steele, 2007; Levy, 2007; Tendler & LaMont, 2011a)
 V. Pertinent Diagnostic Testing. Most diagnostic tests are performed to evaluate other more common causes of abdominal pain and to help rule out other causes.
V. Pertinent Diagnostic Testing. Most diagnostic tests are performed to evaluate other more common causes of abdominal pain and to help rule out other causes.
A. Laboratory Tests (complete chemistry and hematology profiles) to assess for the following:
1. Leukocytosis
2. Hypercoagulable states
3. Shift to immature leukocytes in differential
4. Metabolic acidosis
5. Hyperamylasemia
6. Hemoconcentration
7. Elevated serum lactase
8. Elevated serum phosphate
9. Elevated serum alkaline phosphatase
10. Azotemia
B. Experimental Laboratory Tests Include:
1. Alpha-glutathione s-transferase (Alpha-GST)
2. Intestinal fatty acid–binding protein (I-FABP)
3. Cobalt–albumin binding assay (CABA)
4. Ischemia-modified albumin, a sensitive marker of myocardial ischemia, skeletal muscle ischemia, PE, and stroke; can be used as a diagnostic marker. May have a place in early diagnosis of acute mesenteric embolism (Assar & Zarins, 2008; Broder, 2007; Gunduz et al., 2008; Hugon et al., 2007; McCutcheon, 2009; Tendler & LaMont, 2011a)
C. Noninvasive Tests Include:
1. Endoscopy
a. Little value in diagnosing CMI
b. Often needed to exclude other causes of abdominal pain
c. Contraindicated in SMV with acute symptoms (Cappell, 1998a; McCutcheon, 2009)
2. Sonogram of gallbladder: to exclude gallbladder disease as a source of abdominal pain (Nunnelee, 1999)
3. Intravenous pyelogram: to exclude renal stones as a source of abdominal pain (Nunnelee, 1999)
4. Echocardiography:
a. Used to identify whether the source of embolization is cardiac in origin
b. Useful in postoperative management, especially if anticoagulation is considered (Mansour, 1999)
5. Barium enema/swallow
a. Upper gastrointestinal series may demonstrate nonspecific findings as follows:
1) Malabsorption of contrast segmentation, flocculation, and dilution
2) Bowel dilatation and hypoperistalsis
3) Mural thickening
b. The most common finding of barium enema is thumbprinting:
1) Caused by submucosal edema and hemorrhage
2) Appears as multiple, round, smooth, soft tissue densities projecting into the air-filled colonic lumen
c. Thumbprinting can also occur with the following:
1) Inflammatory bowel disease
2) Pseudomembranous colitis
3) Amebic colitis
4) Intramural hemorrhage from Henoch–Schönlein purpura or anticoagulation therapy
d. Acute findings of barium enema may resemble findings of the following:
1) Diverticulitis
2) Colon cancer
3) Ulcerative, infectious, or Crohn colitis
e. Contraindicated when
1) There is suspected SMV
2) Intraluminal barium contraindicated as it interferes with visualization during angiography (Cappell, 1998b; McCutcheon, 2009; Tendler & LaMont, 2011a)
6. Colonoscopy is required when gastrointestinal bleeding occurs
a. It is preferred over sigmoidoscopy because about 50% of ischemic colonic lesions are proximal to the sigmoid colon.
b. The entire colon is visualized.
c. It is highly important in diagnosing ischemic colitis.
d. It is usually nondiagnostic for CMI (Cappell, 1998b).
7. Plain abdominal roentgenograms:
a. A standard diagnostic tool to evaluate acute abdominal pain
b. Most common findings include the following:
1) Adynamic ileus
2) Associated distention, air–fluid levels, and fixed, dilated loops of bowel
3) Thumbprinting or thickening of bowel loops
c. May see calcification of mesenteric vessels in CMI
d. After infarction the following may be seen:
1) Pneumatosis intestinalis: the presence of thin-walled, gas-filled cysts in the intestines
2) Gas in portal vein
3) Both are signs of poor prognosis
e. A negative radiographic finding in the setting of acute, severe abdominal pain may be the earliest indicator of an ischemic event (Assar & Zarins, 2008; McCutcheon, 2009; Ruotolo & Evans, 1999; Tendler & LaMont, 2011a, 2011b)
8. CT scan: superior to plain films for imaging specific signs of mesenteric ischemia
a. Bowel wall thickening is the most common finding.
b. Relatively specific findings of mesenteric ischemia include the following:
1) Abnormal gas in the bowel wall of portal system
2) Acute embolic infarction of the spleen
3) Thrombi in the mesenteric vessels
4) Absence of bowel wall enhancement
5) Intestinal pneumatosis
c. Gold standard for diagnosing MVT demonstrating
1) Defect in filling or occlusion of SMV
2) Mesenteric congestion
3) Wall thickening in the small bowel
4) Establishes 90% of diagnoses except in cases of early thrombosis in small vessels
5) Magnetic Resonance Angiography (MRA) may be highly sensitive to MVT but CT preferred due to cost and availability (Hirsch et al., 2006; Levy, 2007; McCutcheon, 2009; Tendler & LaMont, 2011a)
9. Doppler ultrasonography
a. A sensitive and specific screening examination for CMI
b. Useful as an alternative to arteriography in evaluating celiac and mesenteric stenosis, 96% accuracy for >70% stenosis
c. May show dilated SMV and presence of collaterals in chronic occlusion with MVT
d. Accuracy of the test requires considerable training and expertise for the ultrasonographer
e. Accuracy may be limited by the following:
1) Respiratory motion
2) Intraperitoneal gas
3) Previous laparotomy
4) Obesity
5) Air-filled loops of distended bowel (Herbert & Steele, 2007; Kougias et al., 2007; McCutcheon, 2009; Tendler & LaMont, 2011a, 2011b)
D. Invasive Tests: Angiography is the gold standard for acute arterial ischemia with digital subtraction angiography the most accurate to determine degree of stenosis
1. Anteroposterior and lateral views are essential for complete arteriographic evaluation to
a. Visualize the collateral arcades
b. Visualize origins of major visceral vessels
c. Exclude other causes of mesenteric ischemia such as MAL syndrome also known as celiac compression syndrome
d. Absence of collateralization is suggestive of an acute process
2. Four reliable arteriographic criteria for the diagnosis of mesenteric vasospasm include
a. Narrowing of the origins of multiple branches of the SMA
b. Alternate dilatation and narrowing of the intestinal branches, the ‘‘string of sausages’’ sign
c. Spasm of the mesenteric arcades
d. Impaired filling of the intramural vessels
3. In MVT, angiography may reveal
a. Reflux of contrast material backs into the aorta due to extremely slow flow and heightened outflow resistance
b. Characteristic sign is a prolonged arterial phase with accumulation of contrast and thickened bowel walls
c. Extravasation of contrast material into the bowel lumen may indicate active bleeding
d. Definitive diagnosis of MVT is made during the venous phase by
1) Filling the defect within the portal vein
2) In extensive thrombosis there is an absence of the entire venous phase (Assar & Zarins, 2008; Bassiouny, 1997; Herbert & Steele, 2007; Kougias et al., 2007; McCutcheon, 2009; McKinsey & Gewertz, 1997; Moawad et al., 1997; Tendler & LaMont, 2011a)
E. Special Procedures
1. Tonometry has been proposed as a useful screening tool for abdominal pain in suspected CMI.
a. Two measurements may identify CMI:
1) A drop in the intramural pH of the jejunum after introduction of a test meal, along with the onset of abdominal pain
2) Increased gastric PCo2 during exercise
2. Endoscopic visible light spectroscopy
a. Abnormally low mucosal oxygen saturations are measured in the proximal small bowel of patients with CMI
b. Measures mucosal capillary hemoglobin oxygen saturation, a direct measure of the adequacy of mucosal perfusion
c. Currently limited to point measurements rather than assessing entire endoscopic field (Friedland, Benaron, Coogan, Sze, & Soetikno, 2007; Herbert & Steele, 2007; Kolkman, Groeneveld, Van der Berg, Rauwerda, & Meuwissen, 1999; Moawad & Gewertz, 1997; Tendler & LaMont, 2011b)
3. Fluorescein-assisted laparoscopy (FAL)
a. The luminescence markedly differentiates the perfused, viable tissue from ischemic tissues.
1) Perfused segments are highlighted with the bright yellow-green dye
2) Ischemic areas remain dark
b. Used to assess early stage acute bowel ischemia and second look 24 to 48 hours after surgery
c. May be laparoscopically which is associated with less postoperative morbidity (Paral et al., 2007)
 VI. Medical Management
VI. Medical Management
A. Therapy Depends on
1. Cause
2. Disease severity
3. Timing of presentation
4. Presence of comorbidity (Cappell, 1998b)
B. Patients are treated with medical therapy unless they present with physical signs or laboratory evidence of peritonitis that requires surgery (Cappell, 1998b; Tendler & LaMont, 2011a)
C. Initial Management Includes:
1. Conditions that decrease mesenteric perfusion are treated
a. Volume resuscitation for hypovolemia
b. Cardiac function optimized
c. Medications that cause mesenteric vasoconstriction are discontinued or avoided
2. Correction of acidosis if possible
3. Administration of appropriate antibiotics
4. Anticoagulation with heparin is initiated to prevent further thrombus propagation
5. Hyperalimentation is initiated for
a. Malnourishment
b. Profuse diarrhea
c. Prolonged clinical course
6. A Foley catheter and peripheral arterial line are placed to monitor intravascular volume and hemodynamic status
7. Nasogastric tube is placed to decrease chances of aspiration and for gastric decompression
8. Narcotics are not initially given because they can blunt the signs of peritonitis
9. Cathartics are contraindicated because they can cause colonic perforation
10. In patients with NOMI, vasodilators such as papaverine may be administered (Assar & Zarins, 2008; Cappell, 1998b; Herbert & Steele, 2007; Mansour, 1999; McKinsey & Gewertz, 1997; Tendler & LaMont, 2011a)
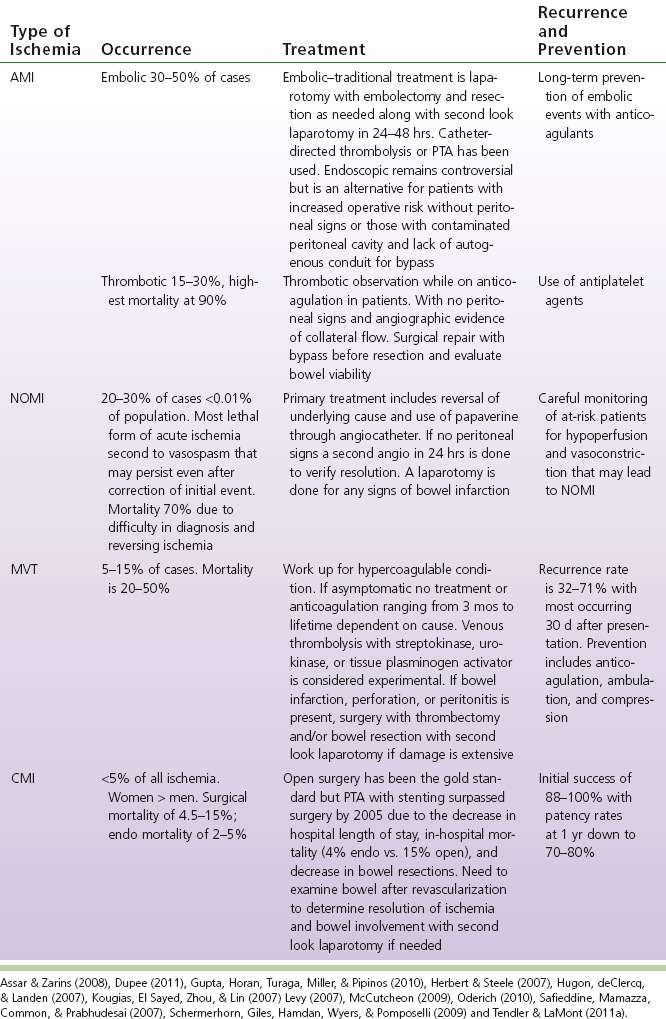
TABLE 12-1 Occurrence, Treatments, Recurrence, and Prevention of Mesenteric Ischemia