CHAPTER 6
Medications Used in Patients with Peripheral Vascular Disease
Lori L. Schirmer
Renee Mosier
First Edition Authors: Sarah E. Connelly and Marjorie Lovell
OBJECTIVES
1. Identify medication classes used to medically manage patients with vascular disease.
2. Describe the mechanism of action for each medication discussed.
3. Discuss appropriate indications for each medication class discussed, specifically relating to vascular disease management.
4. Describe key nursing parameters for monitoring the medications discussed.
Introduction
The medical management of patients with vascular disease involves modification of known atherosclerotic risk factors to slow vascular disease progression, treatment of intermittent claudication (IC) symptoms, prevention of atherothrombotic complications, and treatment of acute thrombotic events. Several classes of medications are used to accomplish the goals of medical management, namely, antithrombotics, cholesterol-lowering medications, vasodilators, nutritional supplements, and antihypertensive agents. This chapter will focus on agents of each medication class and describe how they are used in the management of vascular disease.
Section 1: Lori L. Schirmer
Antithrombotics
Antithrombotic agents include anticoagulant, antiplatelet, and thrombolytic drugs. Each antithrombotic agent will be discussed separately since each has a specific role in vascular disease. The clinical use of dextran will also be discussed because surgeons have used the antithrombotic side effects of this agent to advantage.
Anticoagulants
Commonly used anticoagulants are as follows:
Vitamin K antagonist
• Warfarin (Coumadin)
Direct thrombin inhibitors (DTIs)
• Argatroban
• Dabigatran (Pradaxa)
Factor Xa inhibitors
• Apixaban (Eliquis)
• Edoxaban (investigational use)
• Fondaparinux (Arixtra)
• Rivaroxaban (Xarelto)
Heparins
• Unfractionated heparin (UFH)
• Low molecular weight heparin (LMWH)
Dalteparin (Fragmin)
Enoxaparin (Lovenox)
Nadroparin (Fraxiparin, Fraxiparine) not available in the United States
Tinzaparin (Innohep) not available in the US
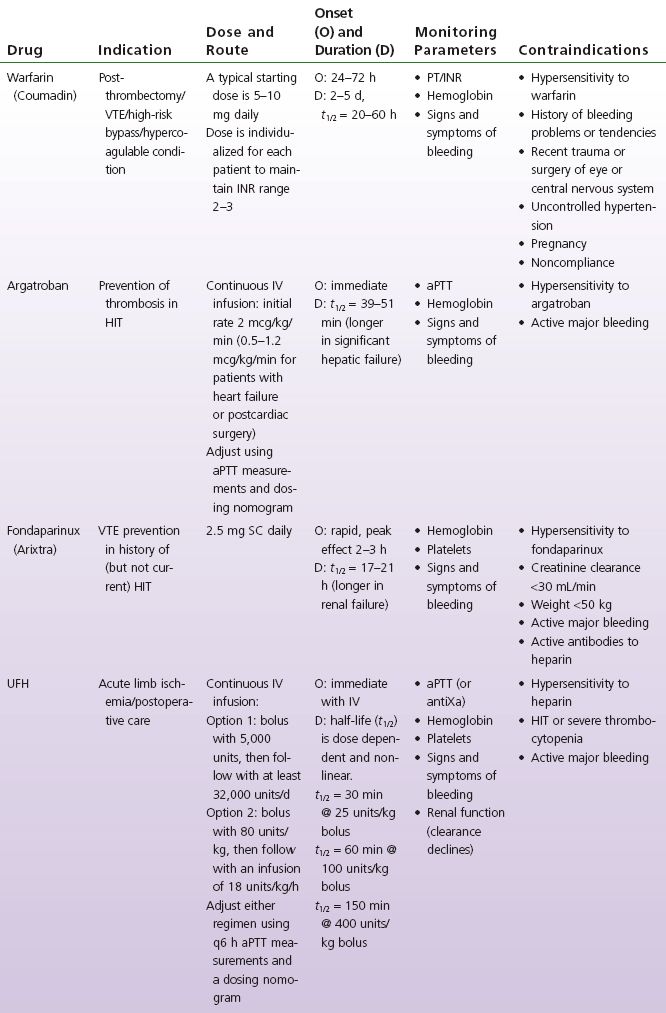
Of these agents, warfarin, dabigatran, apixaban, and rivaroxaban are oral anticoagulants. UFH may be administered intravenously (IV) or subcutaneously (SC). LMWHs and fondaparinux are given SC for peripheral vascular disease (PVD) indications. Argatroban is administered IV.
Mechanism of Action
Warfarin, DTIs, factor Xa inhibitors, and heparins differ slightly in their mechanisms of action, but each works by inhibiting specific coagulation factors, which disrupt the coagulation pathway and ultimately prevent the formation of fibrin, the substance responsible for clot stabilization.
Warfarin
Warfarin inhibits the hepatic conversion of vitamin K epoxide back to vitamin K (Ageno et al., 2012). The result is a reduction in the amount of vitamin K that is available to activate clotting factors II, VII, IX, and X (Ageno et al., 2012). The liver ultimately slows down production of clotting factors when vitamin K is unavailable and the factors that do get synthesized are inactive. The formations of natural anticoagulant proteins C and S are also dependent on vitamin K. The inhibition of protein C (half-life, 8 to 10 hours) and S (half-life, 40 to 60 hours) occurs rapidly compared with the inhibition of clotting factor II (half-life, 60 to 100 hours) and may cause a temporary procoagulant effect within the first 5 days of therapy (Ageno et al., 2012; Haines, Witt, & Nutescu, 2008). This temporary hypercoagulable state means that many patients will receive two anticoagulants (e.g., warfarin plus an LMWH) for the first 5 days of warfarin treatment to prevent clots from forming.
The initial partial suppression of the coagulation pathway may be evident within 24 to 36 hours of therapy and is represented by small elevations in the international normalized ratio (INR). However, full anticoagulation or an antithrombotic effect (the ability to prevent or interfere with thrombi formation) occurs once the formation of all four vitamin K clotting factors has been impaired. The time to full anticoagulation onset is affected primarily by the half-lives of vitamin K clotting factors and is generally not seen until 8 to 15 days of warfarin therapy (Haines et al., 2008). Factor VII production is the first to be inhibited because its half-life is only 6 to 8 hours. Factor II, with a half-life of 60 to 100 hours, is the final coagulation factor inhibited (Haines et al., 2008).
In summary, small elevations in INR may be apparent within 24 hours and the INR may be therapeutic within 5 days; however, full anticoagulation results from the inhibition of all vitamin K clotting factors, which requires up to 15 days of warfarin therapy (Haines et al., 2008). The time to anticoagulation onset and the dose a patient will require to achieve a therapeutic INR are variable due to the following: age, liver metabolism differences determined by genetics, dietary intake of vitamin K containing foods, overall nutritional status, drug interactions, and drug metabolism (Haines et al., 2008).
Direct Thrombin Inhibitors
Argatroban and dabigatran are DTIs and work by binding directly to thrombin (factor IIa) and blocking its action. They are considered to be direct because they do not need to bind to another cofactor to be active, unlike the indirect anticoagulants heparin and LMWHs (Ageno et al., 2012; Garcia, Baglin, Weitz, & Samama, 2012). Argatroban is administered IV and dabigatran is taken orally.
Factor Xa Inhibitors
Apixaban, fondaparinux, and rivaroxaban are factor Xa inhibitors. Fondaparinux is an indirect inhibitor of Xa since it interacts with AT similar to heparin and LMWH (Haines et al., 2008). Apixaban and rivaroxaban are direct inhibitors of factor Xa since they bind to Xa without needing a cofactor like antithrombin (Ageno et al., 2012; Weitz, Eikelboom, & Samama, 2012). Fondaparinux is administered SC while apixaban and rivaroxaban are taken orally.
Unfractionated Heparin
UFH is a mixture of heparin molecules of different sizes. Heparin binds to and potentiates the effect of antithrombin III (AT), a natural anticoagulant. Heparin is considered an indirect anticoagulant because it needs to bind to AT to work. The heparin–AT complex binds to and inactivates thrombin (factor IIa), the coagulation factors IXa, Xa, XIa, and XIIa. The anticoagulant effect is achieved primarily through inactivation of factors IIa and X, because they are more responsive to the heparin–AT complex inhibition (Haines et al., 2008). By blocking thrombin, heparin prevents the formation of fibrin and also inhibits thrombin-induced activation of platelets (Garcia et al., 2012). Unlike warfarin, the anticoagulation effect is rapid. UFH prevents thrombi from expanding and allows the patient’s thrombolytic pathway to degrade the clot. Note that with IV administration, the effect is immediate, and with SC administration, anticoagulation is apparent within 1 to 2 hours (Haines et al., 2008).
Low Molecular Weight Heparins
LMWHs are heparin fragments that are approximately one third the size of a heparin molecule. The mechanism of action of LMWHs is similar to that of UFH in that LMWHs bind to and potentiate the effect of AT. However, because the LMWHs are smaller molecules, the LMWH–AT complex is only capable of binding with and inactivating coagulation factors Xa and IIa. The anticoagulation effect is achieved primarily through factor Xa inhibition. The decreased ability of LMWH to inactivate factor IIa (thrombin) occurs because the majority of the small heparin fragments are unable to bind simultaneously to both AT and factor IIa (Garcia et al., 2012; Haines et al., 2008).
Indications and Evidence
Warfarin
Maintaining vascular surgery graft patency: The administration of long-term warfarin therapy after vascular reconstructive surgery is not recommended because the evidence that it is beneficial is weak and the risk of hemorrhagic complications is high in comparison with the small increase in long-term graft patency (Alonso-Coello et al., 2012). The American College of Chest Physicians recommends that a single antiplatelet agent (discussed below) be used alone instead of adding warfarin for long-term therapy after peripheral artery bypass graft surgery (Alonso-Coello et al., 2012).
Postoperative care (post-thrombectomy): The administration of long-term warfarin therapy may be initiated with IV heparin therapy after a thrombectomy to prevent recurrent embolism. Warfarin therapy is usually maintained for 3 months (Kearon et al., 2012; Norgren et al., 2007). However, it should be noted that long-term (past 3 months) administration of warfarin remains controversial after thrombectomy in the absence of a proven emboli source. The American College of Cardiology Guidelines have recently changed the recommendation from supporting long-term anticoagulation with warfarin in 2008 (Sobel & Verhaeghe) to only recommending it in low or moderate bleeding risk patients in 2012 (Kearon et al.).
Cardiovascular risk reduction: Warfarin is not recommended to be added to antiplatelet therapy (discussed below) for decreasing the risk of cardiovascular ischemic events unless there is another proven indication (e.g., atrial fibrillation) for warfarin (Rooke et al., 2011).
Argatroban and Fondaparinux
Treatment of heparin-induced thrombocytopenia (HIT): HIT is an adverse reaction to heparin or LMWH that results in the formation of antibodies against the drug and can result in severe thromboembolic complications (PE, ischemic limb, stroke, etc.). The role for argatroban in patients with PVD is in treatment and prevention of thrombosis in patients who currently have antibodies to heparin (Linkins et al., 2012). Fondaparinux may be used in patients with a past history of HIT who do not currently produce antibodies but who later (during an event unrelated to the initial episode of HIT) need treatment for or prevention of thrombosis (Linkins et al., 2012).
Apixaban, Dabigatran, and Rivaroxaban
Treatment or prevention of embolism: Oral anticoagulant alternatives to warfarin are a significant advance in patient care because they do not require the laboratory monitoring and frequent adjustments in dosing that warfarin requires. A potential for concern exists however, because an agent that can be used to reverse the anticoagulant effect of these drugs does not exist currently but may be available in the future. The three oral drugs currently approved for use, apixaban, dabigatran, and rivaroxaban are indicated for the treatment of venous thromboembolism and pulmonary embolism and/or prevention of embolic stroke in atrial fibrillation (Ageno et al., 2012; Lexi-Drugs, 2013). Other indications are currently being investigated.
UFH and LMWHs
The rapid onset of action of UFH and LMWHs allows these anticoagulants to be used when an immediate anticoagulant effect is required. A lower rate of recurrence of VTE is associated with achieving therapeutic anticoagulation within the first 24 hours of IV UFH treatment (Garcia et al., 2012). LMWHs are used infrequently in the vascular surgery population primarily because there is a wealth of evidence for the use of UFH and because the elimination half-life of LMWHs is significantly greater compared with UFH. However, a few studies using LMWHs have been completed.
Acute limb ischemia: The administration of IV UFH at a full anticoagulant dose is required immediately following the diagnosis of acute limb ischemia secondary to thromboembolic arterial occlusion to prevent clot propagation and secondary arterial embolus formation. There are no trials comparing UFH to another anticoagulant for this indication and so heparin remains the standard therapy (Alonso-Coello et al., 2012; Norgren et al., 2007).
Postoperative care (post-thrombectomy/thrombolysis): A full anticoagulant dose of IV UFH should be administered to prevent embolic recurrence immediately after a thrombectomy or thrombolysis procedure for the management of acute limb ischemia. Treatment with IV UFH should be followed by warfarin for a minimum of 3 months (Ageno et al., 2012; Norgren et al., 2007).
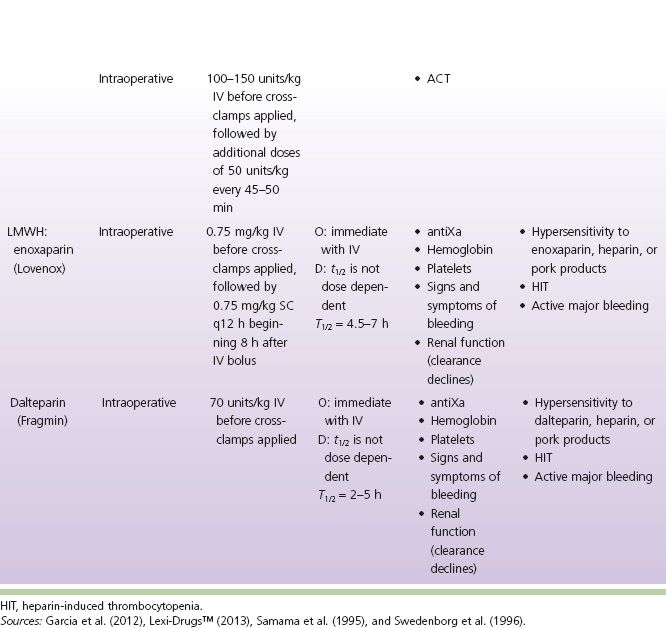
Intraoperative anticoagulation: UFH is used routinely to prevent intravascular coagulation, stasis thrombosis, and accumulation of thrombi during vascular reconstructive procedures (Effeney, Goldstone, Chin, Krupski, & Ellis, 1981; Martin, Greenstein, Gupta, Walker, & Kester, 1994; Sobel & Verhaeghe, 2008; Thompson et al., 1996; Wakefield et al., 1994). Despite the common use of IV UFH prior to clamping arteries, vascular surgeons have not come to a consensus regarding the dose, timing, or method of administration (Wakefield et al., 1994). LMWHs have been shown to be as effective as UFH during infrainguinal bypass surgery (Samama, Gigou, & Ill, 1995; Swedenborg, Nydahl, & Egberg, 1996); however, UFH continues to be the recommended agent for intraoperative use (Sobel & Verhaeghe, 2008) (see Table 6-1).
TABLE 6-1 Antithrombotic Comparison Chart
Antiplatelet Drugs
The available and commonly used antiplatelet drugs are as follows:
• Aspirin
• Clopidogrel (Plavix)
• Ticlopidine (Ticlid)
• Dipyridamole and aspirin (Aggrenox)
• Ticagrelor (Brilinta)—recommended only for coronary patients
Patients with PVD are at high risk for cardiovascular ischemic events. Studies with PVD patients have shown that approximately 50% have symptoms of coronary artery disease (CAD) (or electrocardiographic abnormality); 90% have abnormalities on coronary angiography; and 40% have duplex evidence of carotid artery disease (Tierney, Fennessy, & Hayes, 2000). When all relevant and current trials are summarized, it appears that approximately 60% of PVD patients will have significant atherosclerosis of the cardiac or cerebral arteries (Norgren et al., 2007). The risk of death with symptomatic PVD is approximately 30% and 50% within 5 and 10 years, respectively. The primary cause of death is related to myocardial infarction (40% to 60%) or stroke (10% to 20%) (Norgren et al., 2007). The overall mortality rate among PVD patients, which is caused by a cardiovascular event, is estimated to occur in approximately 80% of cases (Aronow & Ahn, 1994; Dormandy et al., 1989; Hertzer et al., 1984). The primary role of antiplatelet therapy is to reduce a patient’s risk of experiencing a cardiovascular ischemic event (myocardial infarction, stroke, or transient ischemic attack) (Hiatt, 2001; Norgren et al., 2007; Tierney et al., 2000).
Mechanism of Action
Aspirin
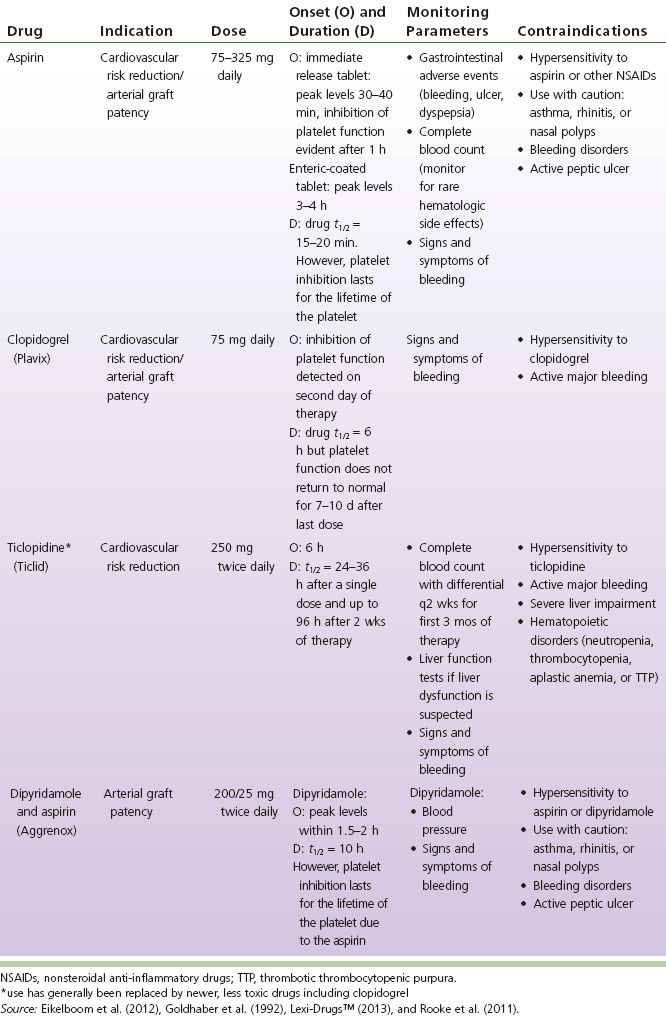
Aspirin irreversibly inhibits the cyclooxygenase (COX) enzyme and thereby prevents the conversion of arachidonic acid to various prostaglandins. Two types of COX enzymes exist: COX-1, which is located predominately within platelets, and COX-2, which is expressed within tissues in response to an inflammatory reaction. The COX-1 enzyme is more sensitive to aspirin inhibition, and it is this enzyme, which is responsible for the production of the prostaglandin thromboxane A2, that causes vasoconstriction and stimulation of platelet aggregation. Low doses (30 to 100 mg) of aspirin are effective at inhibiting COX-1 but are less effective against COX-2. Thus, to prevent platelet aggregation, clinicians may use low doses of aspirin to achieve an adequate response with less gastrointestinal and bleeding adverse effects (Eikelboom, Hirsh, Spencer, Baglin, & Weitz, 2012).
Clopidogrel and Ticlopidine
Clopidogrel and ticlopidine are thienopyridine derivatives, and both are ADP-receptor antagonists. Clopidogrel and ticlopidine are first activated through the cytochrome P450-dependant pathway and subsequently bound with and irreversibly alter receptor P2Y(12) on the surface of platelets. The irreversible change to this receptor antagonizes ADP from binding to platelets and thereby prevents subsequent ADP-mediated platelet aggregation (Eikelboom et al., 2012). The inhibition of ADP platelet aggregation in turn inhibits platelet aggregation induced by other agonists such as thromboxane A2, platelet-activating factor, collagen, and low concentrations of thrombin. However, this additional effect may not be clinically significant (Eikelboom et al., 2012).
Ticlopidine use is associated with significant blood toxicities including neutropenia, thrombocytopenia, and aplastic anemia; therefore, its use is generally reserved for those who cannot take aspirin or clopidogrel (Lexi-Drugs, 2013; Sobel & Verhaeghe, 2008) and is rarely prescribed.
Dipyridamole
Dipyridamole is an agent with vasodilator and antiplatelet actions. Dipyridamole causes accumulation of cyclic AMP (cAMP) within platelets and this leads to platelet inhibition. There are two proposed mechanisms for this buildup of cAMP. One is the inhibition of the enzyme (cyclic nucleotide phosphodiesterase) that degrades cAMP. The second mechanism is blockade of platelet uptake of adenosine which further contributes to increased cAMP. The vasodilator properties may then result from the direct stimulation of prostaglandin I2 synthesis and protection against its degradation (Eikelboom et al., 2012).
Dipyridamole alone has not produced favorable clinical results. However, a modified-release dipyridamole in combination with low-dose aspirin (Aggrenox) has been shown to significantly reduce the risk of stroke in patients who had previously experienced a cerebral ischemic event compared with patients who received aspirin alone (Eikelboom et al., 2012).
Ticagrelor
Ticagrelor is a non-thienopyridine (ADP) receptor antagonist that inhibits platelet aggregation. “Tricagrelor completely inhibits ADP-induced platelet inhibition ex vivo through reversible binding reversibly to the P2Y12 receptors” (Jennings, 2010). Tricagrelor does not require metabolic activation in order to inhibit platelets.
Indications
Aspirin
To reduce peripheral vascular graft occlusion in patients with prior bypass surgery
To reduce the need for peripheral arterial surgery
To reduce the risk of cardiovascular ischemic events
Clopidogrel and Ticlopidine
To reduce peripheral vascular graft occlusion in patients with prior bypass surgery
To reduce the risk of cardiovascular ischemic events
Dipyridamole and Aspirin
To reduce peripheral vascular graft occlusion in patients with prior bypass surgery. [Note that the dose of aspirin in the combination product Aggrenox is 25 mg and may not be adequate to prevent recurrent myocardial infarctions or angina attacks (Lexi-Drugs, 2013.)
Ticagrelor
Ticagrelor is indicated to reduce the rate of thrombotic cardiovascular events in patients with acute coronary syndrome (ACS) (unstable angina, non-ST elevation myocardial infarction, or ST elevation myocardial infarction (Wallentin et al., 2010). Ticagrelor should be used in combination with aspirin.
Evidence
Prevention of Arterial Graft Occlusion
Aspirin and dipyridamole: A metaanalysis conducted by Tangelder, Lawson, Algra, and Eikelboom (1999) of five randomized controlled trials (Donaldson et al., 1985; Goldman, & McCollum, 1984; Green, Roedersheimer, & DeWeese, 1982; Kohler et al., 1984; McCollum, Alexander, Kenchington, Franks, & Greenhalgh, 1991) demonstrated a pooled overall treatment effect favoring the use of aspirin and dipyridamole for preventing graft occlusion. Only one of the five studies examined the use of aspirin alone compared with placebo (Green et al., 1982). The pooled relative risk (RR) across the five trials for bypass occlusion was 0.78 (95% CI, 0.64% to 0.95%) or a risk reduction of approximately 22% (Tangelder et al., 1999). A review performed by the Antiplatelet Trialists’ Collaboration (ATC) group (ATC, 1994) supported the findings of Tangelder et al. (1999). The ATC group concluded that antiplatelet therapy with aspirin significantly improved vascular graft patency in 3,226 patients with PVD who were treated with bypass surgery (saphenous vein or prosthetic graft) or peripheral angioplasty (ATC, 1994). There was a statistically significant 43% proportional reduction in vascular graft occlusion, from 25% in the control group to 16% in the antiplatelet group (ATC, 1994). Despite these positive results, caution should be exercised when interpreting the findings because the trials following the bypass procedures were small (a total of 423 patients in the active group and 393 patients in the control group) and of a relatively short duration (1-year follow-up). Ultimately the question of whether or not there is sufficient evidence of value of using aspirin with dipyridamole to prevent graft occlusion is somewhat irrelevant since all patients with symptomatic PVD who do not have a contraindication are recommended to take aspirin for the prevention of cardiovascular ischemic events (Alonso-Coello et al., 2012; Sobel & Verhaeghe, 2008).
Ticlopidine: A single clinical study currently supports the use of ticlopidine for the prevention of bypass occlusion in PAD patients undergoing infrainguinal surgery. Becquemin (1997) randomized 243 patients who underwent femoropopliteal or femorotibial saphenous vein bypass graft to either ticlopidine or placebo and followed them for an average of 2 years. Cumulative graft patency rate at 2 years’ follow-up was 82% and 63% for the ticlopidine and placebo groups, respectively (Becquemin, 1997). Despite the clinical evidence, which supports the benefits of ticlopidine on late peripheral bypass occlusion, the use of ticlopidine has been discouraged because of the risk of hematologic side effects (Eikelboom et al., 2012).
Clopidogrel: Evidence to support the use of clopidogrel after peripheral arterial bypass surgery is limited. The CASPAR trial evaluated clopidogrel plus aspirin versus aspirin alone in patients after below knee bypass grafting (Belch & Dormandy, 2010). There was no significant difference in the composite primary outcome of graft occlusion, amputation, or death in the overall patient population, but there was a significant benefit of the addition of clopidogrel to aspirin in the subgroup that had a prosthetic graft (Belch & Dormandy, 2010). The combination of clopidogrel and aspirin is now recommended for 1 year (the average follow-up time in the study) after bypass surgery in those with a prosthetic graft (Alonso-Coello et al., 2012).
Reducing Risk for Peripheral Arterial Surgery
Aspirin: The Physicians’ Health Study (Goldhaber et al., 1992) was designed as a primary prevention trial for patients who had no history of myocardial infarction, stroke, or transient cerebral ischemia. Treatment with aspirin, 325 mg every other day, produced a significant reduction in the need for peripheral arterial surgery (RR of peripheral artery surgery in the aspirin arm was 0.54, p < 0.03). The study, however, showed no difference between aspirin and placebo treatment in the prevention of IC (Goldhaber et al., 1992).
Prevention of Cardiovascular Events
Antiplatelets (aspirin and dipyridamole): The ATC group demonstrated that in patients who are at a higher risk for experiencing vascular events (i.e., those with a history of unstable angina, acute myocardial infarction, prior myocardial infarction, stroke, or transient ischemic attack, and patients with other evidence of vascular disease such as PVD), antiplatelet therapy significantly reduced the odds ratio of experiencing a myocardial infarction, stroke, or subsequent death from a vascular event by 27%. When patients with PVD were examined separately (those with IC, peripheral grafts, or peripheral angioplasty), the benefit of antiplatelet treatment was similar in magnitude but was nonsignificant (ATC, 1994).
While the evidence supporting aspirin for secondary prevention of cardiovascular events is clear in those with symptomatic PVD, it is unclear and controversial if those without symptoms (and therefore in need of primary prevention) benefit from aspirin. The Antithrombotic Trialists’ Collaboration attempted to answer the question of primary prevention benefit in asymptomatic patients with PVD (Baigent et al., 2009). In their metaanalysis of trials with low-dose aspirin they found a significant reduction of nonfatal MI (0.18% vs. 0.23% per year, p < 0.0001), a nonsignificant difference in stroke rates, and a nonsignificant difference in vascular mortality (Baigent et al., 2009). There was also a significant increase in major gastrointestinal and extracranial bleeding (0.10% vs. 0.07% per year, p < 0.0001) that tempers the benefit of a reduced risk of MI (Baigent et al., 2009) in those without symptoms of PVD. The latest ACCP clinical practice guidelines recommend aspirin 75 to 100 mg daily in asymptomatic PVD (Alonso-Coello et al., 2012). The American College of Cardiology Foundation and American Heart Association (ACCF/AHA) practice guideline for peripheral artery disease has not recommended aspirin due to “usefulness not well established” in asymptomatic patients using the same evidence (Rooke et al., 2011). The TASC II (Trans-Atlantic inter-Society Consensus) document for management of peripheral artery disease has taken a neutral stance on the question with their recommendation that aspirin “can be considered” in those without symptoms (Norgren et al., 2007).
Aspirin versus clopidogrel: The study clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE Steering Committee, 1996) directly compared the effects of aspirin and clopidogrel in reducing the risk of ischemic events in patients who had recent myocardial infarctions, ischemic strokes, or PVD. Overall, clopidogrel was found to be significantly more effective in reducing the combined endpoint of ischemic stroke, myocardial infarction, and death from a vascular cause (RR reduction 8.7%, p < 0.043). When PVD patients were examined separately, clopidogrel reduced the average ischemic event rate significantly more compared with aspirin, 4.86% and 3.71%, respectively (RR reduction 23.8%, p < 0.0028) (CAPRIE Steering Committee, 1996).
Aspirin versus clopidogrel and ticlopidine: A review of the Cochrane database for studies using ticlopidine and clopidogrel versus aspirin in high-risk vascular patients determined that ticlopidine and clopidogrel reduced the odds of stroke, myocardial infarction, and vascular death by approximately 9% compared with aspirin therapy. Although ticlopidine and clopidogrel therapy were shown to be significantly more effective than aspirin in preventing vascular events, the magnitude of this benefit was small and must be weighed against adverse effects and cost. Ticlopidine usage has diminished and is discouraged because the risks of complications, such as neutropenia and thrombocytopenia, are significant compared with clopidogrel or aspirin therapy. Clopidogrel use is associated with a greater incidence of diarrhea and skin rash and is significantly more expensive than aspirin. Aspirin is associated with more gastrointestinal intolerance and bleeding (Hankey, Sudlow, & Dunbabin, 2000).
Ticagrelor: The PLATelet inhibition and patient Outcomes (PLATO) trial showed Ticagrelor to be more effective than clopidogrel for the continuous prevention of ischemic events, stent thrombosis, and death in the acute and long-term treatment of patients with ACS with no change in the overall risk of major bleeding (Wallentin et al., 2010). Ticagrelor was approved by the FDA in July 2011. However, there is no data on its use in patients with peripheral artery disease.
Summary
Aspirin or clopidogrel may be used long term in patients after bypass graft surgery to maintain patency. Patients who have a prosthetic graft are recommended to take both aspirin and clopidogrel for the first year after surgery (Alonso-Coello et al., 2012).
Aspirin remains the drug of choice for cardiovascular risk reduction though clopidogrel is an alternative that can be used in symptomatic patients. Although clinical trials have demonstrated clopidogrel to be more effective in reducing cardiovascular events in patients with PVD, the magnitude of this benefit is small, and the cost of clopidogrel is significantly more compared with aspirin (Hankey et al., 2000).
Ticlopidine therapy has dropped out of favor for clinical use because it is associated with a greater incidence of neutropenia and thrombocytopenia (Eikelboom et al., 2012; Hankey et al., 2000).
The combination product dipyridamole and aspirin is not recommended for the prevention of cardiovascular events in patients with PVD. However, it is an appropriate and effective therapy for secondary stroke prevention in patients who have failed aspirin therapy (Lexi-Drugs, 2013).
Unless there is a contraindication to aspirin therapy, every patient with symptomatic PVD should be taking aspirin (the majority of patients) or clopidogrel (those who have an allergy or intolerance to aspirin or who have an unusually low bleeding risk). Some patients with asymptomatic PVD will be candidates for aspirin for primary prevention. After surgical intervention and placement of a prosthetic graft, patients should be considered for both aspirin and clopidogrel for 1 year and then continue one of the two drugs long term (Alonso-Coello et al., 2012; Norgren et al., 2007; Rooke et al., 2011) (see Table 6-2).
Thrombolytics
The thrombolytics that have been used in vascular surgery include the following:
Streptokinase (SK) (Streptase)
Urokinase (UK) (Abbokinase—retired brand name, may return to market as Kinlytic in the future)
Alteplase (recombinant tissue plasminogen activator [rt-PA], Activase)
Reteplase (Retavase)
Tenecteplase (TNKase)
TABLE 6-2 Antiplatelet Comparison Chart
Streptokinase is no longer manufactured and will not be discussed in detail. Urokinase is not currently available since Abbokinase is no longer manufactured and Kinlytic is not yet FDA approved. The use of urokinase will be discussed here because it may become available in the future. The thrombolytics currently available do not have FDA-approved uses for vascular disease indications with the exception of alteplase for treatment of PE or stroke but off-label use is common.
Mechanism of Action
Refer to Table 6-3, Thrombolytic Comparison Chart.
Indications
Thrombolytic drugs may be used intravenously as in treatment of acute MI or acute ischemic stroke. Thrombolytics are used more commonly in patients with PVD in catheter-directed thrombolysis or with mechanical thrombectomy. Heparin is often used concurrently to prevent thrombus propagation (Lew & Weaver, 2010). Table 6-4 indicates the contraindications to thrombolytic therapy that help to determine whether a patient is a candidate for thrombolysis.
TABLE 6-3 Thrombolytic Comparison Chart