Chapter 28 MEDICATIONS
PHARMACOLOGY
‘Pharmakon’ is the Greek word for drug, and pharmacology is the study of the actions, uses and adverse effects of drugs. The term drug has several definitions, which include it being any substance that may be used medicinally in a range of forms and administered to the body by different methods to prevent, diagnose or treat a disease or condition, or ‘any natural or synthetic substance that alters the physiological state of a living organism’ (Taylor & Reide 1998). Furthermore, drugs can be divided into medicinal drugs (or medications), which are substances used in the treatment, prevention and diagnosis of a disease; and non-medicinal drugs (or social drugs), which are substances used for recreational use and include caffeine, alcohol, nicotine, cannabis, heroin and cocaine. The distinction between the two is not always clear-cut, as some non-medicinal substances can be used in a medicinal way (e.g. caffeine is included in some preparations to treat migraine) and some medicinal substances can be used in non-medicinal ways (e.g. opioid analgesics such as pethidine and morphine can be used recreationally for their mind-altering properties).
DRUG FORMULATIONS AND ADMINISTRATION ROUTES
A drug may be presented in a variety of forms (Table 28.1) and administered via several different routes.
| Capsule | Gelatine container enclosing a drug in liquid, powder or granule form |
| Tablet | A drug mixed with a base compound and compressed into a variety of shapes. Tablets are sometimes coated, which delays release of the drug until the tablet reaches the intestine. Tablets are coated if the drug could cause gastric irritation or if it would be destroyed by gastric juice. Another form of tablet is ‘slow release’ (or sustained release), which contains a drug that is released over a prolonged period |
| Granules | Small rounded pellets that are usually coated |
| Lozenge | Small tablet containing a medicinal agent in a flavoured fruit or mucilage base, which dissolves in the mouth to release the drug |
| Mixture | Aqueous vehicle in which drugs are dissolved or suspended |
| Suspension | Liquid in which insoluble particles of a drug are dispersed |
| Elixir | Sweetened, flavoured alcoholic solution containing a drug |
| Linctus | Sweetened syrup containing a drug |
| Tincture | Alcoholic solution containing a drug |
| Emulsion | Mixture of oil and water containing a drug |
| Syrup | Concentrated sugar solution containing a drug |
| Cachet | Envelope of rice paper that encloses a drug |
| Injection | Sterile aqueous or oily solutions and suspensions containing a drug, which are administered parenterally |
| Suppository | Solid preparation containing a drug, which melts when inserted into the rectum |
| Pessary | Solid preparation containing a drug, which is administered vaginally |
| Drops | Aqueous or oily solution containing a drug. Drops may be instilled into the eye, ear or nose |
| Cream | Aqueous or oily emulsion for topical application |
| Ointment | Semi-solid greasy preparation for topical application |
| Paste | Similar to ointment but contains a high proportion of powders. Pastes have a very stiff consistency and will adhere to lesions at body temperature |
| Liniment | Oily or alcoholic preparation for topical application |
| Paint | Liquid preparation for application to the skin or mucous membranes |
| Lotion | Aqueous, alcoholic or emulsified vehicle for topical application |
| Powder (dusting) | Medicated substance for topical application |
Routes of administration of drugs include:
PHARMACOKINETICS
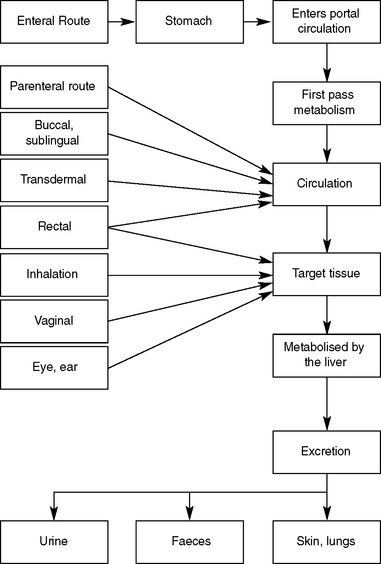
Pharmacokinetics describes the way the body affects the drug over time and relates to the processes of absorption, distribution, metabolism and excretion (Figure 28.1).
ABSORPTION
DISTRIBUTION
After the drug has been absorbed into the general circulation it will then be distributed to different tissues for drug action to occur. To be transported in the blood, the drug molecules become protein bound (i.e. bind loosely to blood proteins); however, there is always some unbound (or free) drug. Equilibrium exists between the bound and unbound drug but only the unbound drug molecules are able to bind with the tissue (Figure 28.2).
The blood plasma contains a variety of plasma proteins (e.g. albumin, corticosteroid-binding globulin (CBG) and glycoproteins), which are able to bind to drugs to produce a drug–protein complex. If plasma proteins are deficient, for example, if a client has a condition such as liver disease, malnutrition or extensive burns, a greater portion of the drug remains unbound and is available to bind to the tissue, increasing the effects of the drug and requiring a decrease in the dose. Conversely, if there is an increased level of plasma proteins, in a client who has multiple myeloma, for example, there is an increase in the amount of bound drug, reducing the drug’s effectiveness, requiring an increase in dose.
Drugs may also compete for the same binding site on the plasma protein when more than one drug is administered concurrently. The drug with the higher affinity (or greater attraction) will be bound and displace the other(s) from the protein-binding site, resulting in the plasma concentration of the now unbound drug increasing. For example, aspirin will displace phenytoin from the plasma protein, resulting in an increased level of unbound phenytoin and, potentially, phenytoin toxicity. The effect is the same as giving an increased dose of the drug (Figure 28.3).
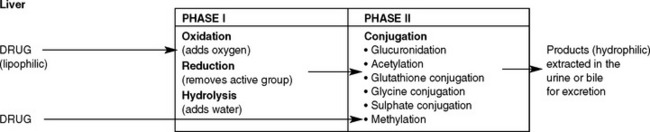
METABOLISM
Normally, these enzymes are present in small amounts. However, in some circumstances the amount of enzymes can alter, thereby also altering the rate of metabolism. For example, alcohol stimulates production of hepatic enzymes in habitual drinkers, resulting in the alcohol being more rapidly metabolised than in a non-drinker. This process of causing the amount of enzymes to be increased is called enzyme induction. Alcohol can also cause the levels of other enzymes involved in drug metabolism to increase, leading to the drug being more rapidly metabolised and therefore having a reduced therapeutic effect. Enzyme induction can be due to environmental pollutants, including benzopyretics in cigarette smoke, pesticides and some drugs, such as warfarin and phenytoin. Enzyme inhibition is the opposite effect and is the decreased synthesis of enzymes, resulting in the slowing down of the metabolic steps and an increased therapeutic effect. Some drugs are known enzyme inhibitors and include cimetidine, erythromycin, diltiazem, verapamil and ketaconazole. Figure 28.4 illustrates the process of drug metabolism simplified.
DRUG EXCRETION
Some drugs, penicillin for example, exit unchanged in the urine, while others must undergo transformation in the liver before being excreted by the kidneys. Many drugs enter the hepatic circulation to be broken down by the liver and excreted into the bile and then into the bowel. The liver has a large metabolic capacity so, in people with liver disease, drug elimination is generally not affected until a large portion of the liver’s functional capacity is lost.
IMPORTANCE OF THERAPEUTIC DRUG MONITORING
For drugs to be therapeutic, a certain blood level needs to be reached and maintained. It is therefore a priority that medications are administered on time. If they are administered late, the level of the medication in the blood may drop below a therapeutic level; for example, if the level of an antibiotic is too low it provides an opportunity for microorganisms to multiply. If the blood levels are too high, toxicity and serious consequences might occur. The main aim of therapeutic drug monitoring is to optimise drug therapy by achieving adequate drug levels, while minimising toxicity. This is especially important in clients at the extremes of age. Clinical Interest Box 28.1 outlines some signs and symptoms of toxicity commonly seen in older clients. Clinical Interest Box 28.2 identifies changes related to ageing that influence pharmacokinetics.
CLINICAL INTEREST BOX 28.1 Indicators of drug toxicity in older adults
(Ebersole & Hess 2005: 295)
CLINICAL INTEREST BOX 28.2 Changes related to ageing that influence pharmacokinetics
PHARMACODYNAMICS
DRUG ACTION
A drug may produce more than one effect:
NURSING CARE AND ADMINISTRATION OF MEDICATIONS
The nurse also requires certain skills to:
LEGAL ASPECTS OF DRUG ADMINISTRATION
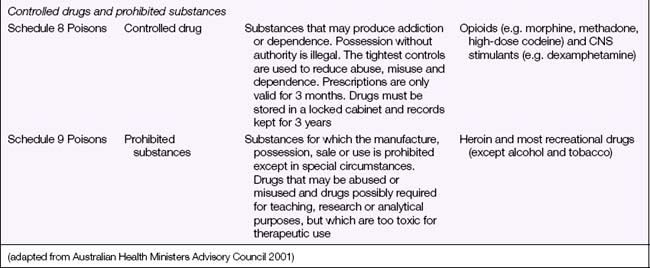
Before a drug can be administered safely, the nurse needs to be aware of the legal aspects of drug administrations. This includes knowledge of the laws governing the possession, use and dispensation of drugs and of the directives of the nurse’s registering body on the administration of medications to clients. It also means observing the employing health care facility’s occupational health and safety (OHS) regulations that are designed to promote safe storage, handling and use of drugs (see Chapter 26). Currently, the states and territories of Australia differ on the regulations governing administration of medication by an Enrolled Nurse (EN). In Tasmania an EN whose qualifications have been determined as appropriate for the purpose of administration of medications and whose practising certificate has been endorsed may administer medications listed in schedule 2, 3 and/or 4 of the Poisons List Order 2001 (Nurses Board of Tasmania 2007). In Queensland, the Health (Drug & Poisons) Regulation 1996 (section 58A) was amended in 2006 to enable the EN with medication endorsement to administer controlled Schedule 8 drugs (Controlled Drugs) under the supervision of a Registered Nurse (RN) or doctor (Queensland Nursing Council 2006). Other states and territories are currently moving towards, or have established, specific guidelines regarding limited medication administration by the EN. Each EN will need to ascertain the regulations specific to the state or territory of Australia in which nursing is practised.
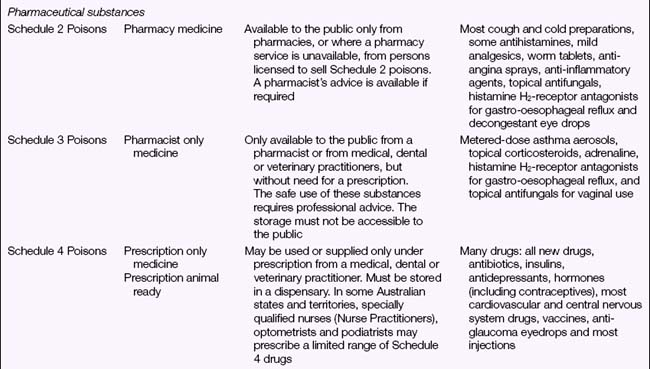
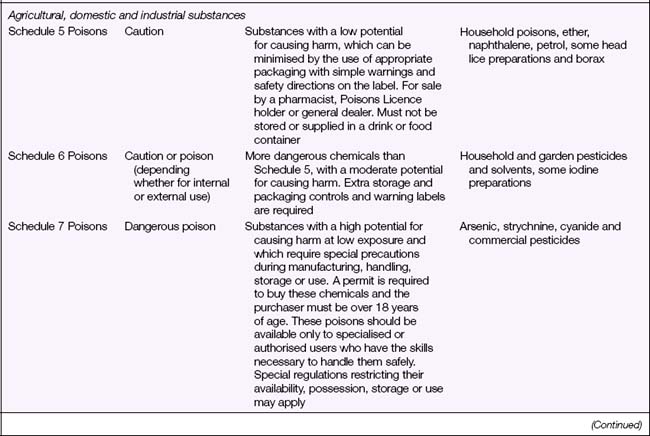
Legal Acts concerning poisons and the poisons regulatory bodies in New Zealand and each state and territory in Australia (Table 28.2) deal with the control of all drugs, from prescription medication through to agricultural poisons and research drugs. The laws and regulations apply to sale, supply, storage, dispensing and labelling. The drugs and poisons schedules divide drugs into groups according to their mode of action, therapeutic use, potency, potential for abuse and addiction, and safety. While there is currently no national drugs and poisons schedule in Australia, and each state and territory has its own version, the recommendations of the National Drugs and Poisons Schedule Committee in the form of the Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP, Table 28.3) are usually incorporated into the legislation and regulations of each state and territory. An agreement between Australia and New Zealand has led to harmonisation of trans-Tasman scheduling, and the two countries now have compatible schedules, labelling and packaging requirements (Bryant & Knights 2006).
TABLE 28.2 AUSTRALIAN LEGISLATION INVOLVED IN THE REGULATION OF DRUGS
| Jurisdiction | Drug regulation legislation | Additional drug offences Acts |
|---|---|---|
| Commonwealth (Cth) | ||
| Australian Capital Territory (ACT) | ||
| New South Wales (NSW) | Drug Misuse and Trafficking Act 1985 (NSW) | |
| Northern Territory (NT) | Misuse of Drugs Act 1990 (NT) | |
| Queensland (Qld) | Drugs Misuse Act 1986 (Qld) | |
| South Australia (SA) | Drugs Act 1908 (SA) | |
| Tasmania (Tas) | Misuse of Drugs Act 2001 (Tas) | |
| Victoria (Vic) | ||
| Western Australia (WA) | Misuse of Drugs Act 1981 (WA) |
ADMINISTRATION OF DRUGS
Before administering medications in any form or by any route the nurse must check that the client does not have any drug allergies. The nurse also observes the client who is starting a new medication for any signs of allergy or adverse reaction, and reports and records such responses promptly to the medical officer and the nurse in charge. If the situation arises that a client chooses not to take a prescribed medication, this must be recorded on the client’s medication chart and also reported to the client’s medical officer and the nurse in charge. The principles of asepsis (see Chapter 25) are employed during the preparation and administration of all medications.
To ensure that medications are administered correctly and safely, the nurse must observe the seven rights of drug administration (Reiss, Evans & Broyles 2002):
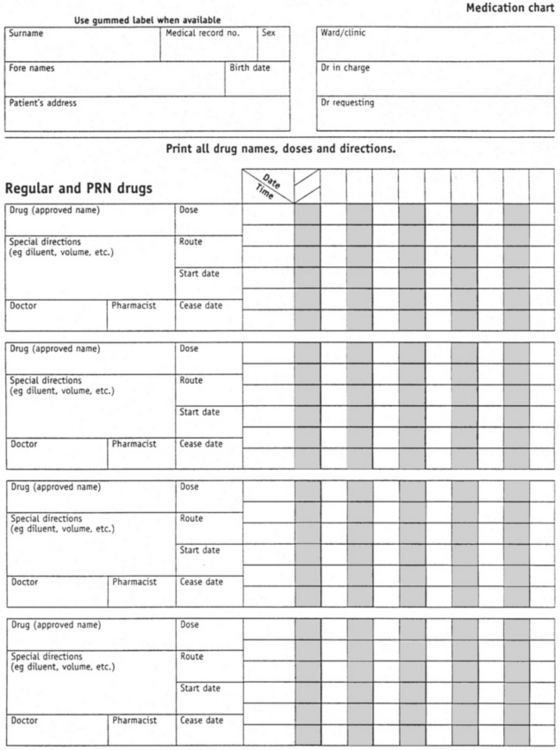
Before any medication is administered the client medication chart (Figure 28.5) must be checked thoroughly and systematically to determine the name of the drug, the route, the dosage, and the date and time for administration of the medication prescribed.
The nurse compares the label of the drug container with the client medication chart three times:
To identify a client correctly, before a drug is administered, the nurse:
ERRORS IN ADMINISTRATION
Errors can arise for many reasons. Information on ways to avoid errors in administration is provided throughout this chapter, and also in Clinical Interest Box 28.3. Each health care facility has its own protocol for dealing with medication errors, and the nurse must understand and adhere to that protocol.
CLINICAL INTEREST BOX 28.3 Safe medication management guidelines
If the nurse does make a medication error (e.g. administering the wrong drug, wrong dose or via the wrong route) or if a nurse identifies an error made by another nurse, the incident must be reported immediately to the nurse in charge. The nurse has a professional and ethical responsibility for reporting any error, no matter how minor or trivial it may seem at the time. Measures to counteract the effects of the error may be necessary, such as administering an antidote (with a medical officer’s order) or monitoring the drug’s effects over time. The nurse is also responsible for completing an incident report form describing the nature of the incident. The incident report provides an objective analysis of why the incident occurred and is a means for the facility’s safety personnel to monitor such events and to implement measures to prevent recurrence. The incident, measures taken and outcomes should also be recorded in the client’s medical notes (see Chapter 20 for information on nursing documentation).