Overview of nutrition
Carbohydrates
Proteins
aConditionally essential or essential only at certain ages or in certain conditions.
Essential Amino Acids
Conditionally Essential Amino Acids
Non-Essential Amino Acids
Lysine
Cysteine
Alanine
Threonine
Tyrosine
Glutamic acid
Histidine
Arginine
Aspartic acid
Isoleucine
Citrullinea
Glycine
Leucine
Taurinea
Serine
Methionine
Carnitine
Proline
Phenylalanine
Glutamine
Tryptophan
Asparagine
Valine
Fat
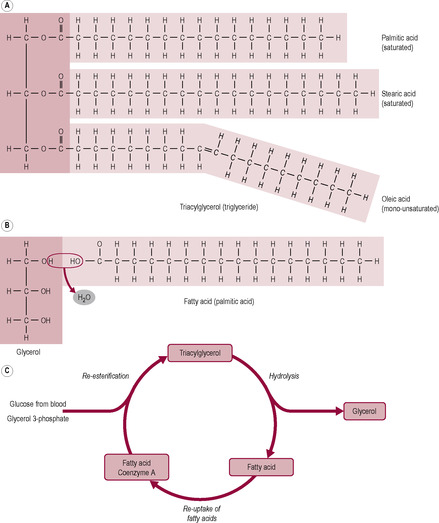
Fig. 12.1
Vitamins
Vitamin
Role
Source
Thiamin (B1)
Carbohydrate metabolism
Pork, wheat germ, yeast
Riboflavin (B2)
Protein metabolism
Offal, milk, grains, legumes, eggs, vegetables
Niacin (B3)
Production of energy from glucose; synthesis of fatty acids
Meat, nuts, legumes
Pyridoxine (B6)
Synthesis and catabolism of amino acids; synthesis of antibodies and neurotransmitters
Pork, offal, grains, legumes, potatoes, bananas
Cyanocobalamine (B12)
Reactions preceding use of folic acid in DNA synthesis
Animal and dairy products, eggs, yeast
Folate
Formation of DNA
Liver, green leafy vegetables, kidney beans, oranges, melon
Pantothenic acid
Metabolism; synthesis of acetylcholine
Liver, egg yolk, milk, dried and spouting beans
Biotin
Synthesis of fatty acids, amino acids and purines (required for DNA and RNA)
Offal, egg yolk, tomatoes
C
Collagen formation, tissue formation and integrity, antioxidant, iron absorption
Citrus fruit, tomatoes, other fruit and vegetables
Vitamin
Role
Source
A
Visual perception (rhodopsin synthesis); growth of epithelial tissue and bones, antioxidant
Liver, kidney, egg yolk
D
Hormone involved in bone mineralization and calcium homeostasis
Synthesized in skin, fish oils
E
Tissue growth + integrity of cell membranes; antioxidant
Vegetable oils, grains, milk, eggs, fish, meat
K
Synthesis of blood-clotting factors; bone metabolism
Gut flora, liver, green leafy vegetables
Minerals
Mineral
Function
Dietary Source
Sodium (Na)
Extracellular ion essential for the generation of action potentials; required in the active transport of small molecules into the cell
Table salt (NaCl)
Potassium (K)
Intracellular ion essential for the generation of action potentials; utilized by the cell to maintain ion concentration gradients
Meat, milk, fruits, vegetables
Calcium (Ca)
Bone and teeth structural component; essential for blood clotting, muscle contraction and nerve impulse conduction
Dairy products, fortified flour, cereals, green vegetables
Chlorine (Cl)
Cation in body fluids; gastric acid excretions
Salt (NaCl)
Phosphorus (P)
Structural component of bones and teeth; essential for formation of ATP for energy storage
Meat, dairy products cereals, bread
Magnesium (Mg)
Required by some enzyme activities; present in cells, body fluids and bone
Vegetables, milk, cereals, bread
Iron (Fe)
Transfer of oxygen in haemoglobin molecule; oxidation processes; electron transfer chain
Meat, vegetables, flour
Zinc
Enzyme activity; growth and development of the immune system; spermatogenesis; tissue growth
Oysters, steak, crab meat, red meat, milk products
Iodide
Thyroid hormones
Seafood, iodized table salt
Copper
Constituent of enzymes; energy production and release
Legumes, grains, nuts and seeds, offal
Manganese
Synthesis of urea; conversion of pyruvate in TCA cycle
Plant products
Fluoride
Essential to reduce decay in bone and tooth tissues
Fluoridated drinking water
Chromium
Carbohydrate and lipid metabolism
Unrefined foods, brewer’s yeast, whole grains and nuts
Selenium
Antioxidant; catalyst for the production of thyroid hormone
Liver, shellfish, fish meat
Preconceptual nutritional status
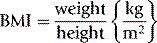
Grade
BMIa
Definition
–
<20
Underweight
0
20–24.9
Desirable weight
I
25–29.9
Overweight
II
30–39.9
Mild obesity
III
40
Severe obesity
aNormal: 19.8–26.0.
Non-nutritional factors affecting reproductive function
Listeriosis
Salmonellosis
Toxoplasmosis
Campylobacters
Nutritional requirements in pregnancy
Energy requirements
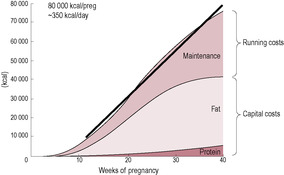
Fig. 12.3
Protein requirements
Fat requirements
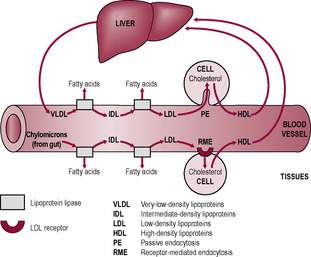
Fig. 12.4
Carbohydrate requirements
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Maternal nutrition and health
Both Zara and James follow a vegetarian diet and try to lead a healthy lifestyle. At her first visit to the midwife, Zara was calculated as having a body mass index (BMI) of 24, which the midwife calculated from what Zara reported as her non-pregnant weight. During the pregnancy, Zara’s baby appeared to be growing as expected and her midwife has measured Zara’s uterine growth in centimetres using the symphysis pubis as the reference point; as expected, the fundal height has increased by 1 cm per week of pregnancy.
At 34 weeks; gestation, Zara is concerned that she only seems to have gained 4.5 kg over her non-pregnant weight unlike her sister who has put on over 10 kg and has been told by her midwife that her baby is a little bit on the small side.
• What factors could explain the differences in weight gain between Zara and her sister?
• What are the benefits of calculating the BMI of pregnant mothers and why is it preferable to calculate the BMI using the non-pregnant values if they are available?
• Does Zara’s smaller weight gain give any cause for concern?
• What other reasons could explain why Zara’s sister appears to have gained a lot more weight than Zara has?
Growth, development and optimal health rely on good nutrition and an adequate quality and quantity of nutrients for the cells. However, diet is influenced by many factors including wealth, religion, culture, and geographical and social factors. The insoluble macromolecules of food must be digested into soluble and absorbable subunits (see Chapter 1). The major components of the diet, or macronutrients, are carbohydrates, proteins and fats. Essential micronutrients are vitamins and minerals. Water is also an essential part of the diet.
Carbohydrates are the major energy source in the majority of human diets, but the amount and type of carbohydrate consumed varies amongst different population groups. With increased affluence in the Western world, there is a tendency to increase the proportion of fat in the diet at the expense of carbohydrate. There are two major types of carbohydrate: polysaccharides (or complex carbohydrates) and simple sugars (monosaccharides and disaccharides).
Monosaccharides, such as glucose, fructose and galactose, are not usually consumed in high quantities although they do occur in fruit. The major source of carbohydrate in the diet is usually starch from plant sources, plus some glycogen from animal liver and muscle. Dietary disaccharides include sucrose (table sugar), lactose (in milk) and maltose, which occurs in malt, beer and some sprouting seeds. Most starchy foods are high in carbohydrate and low in fat. With increasing affluence, added sugars tend to contribute more to the carbohydrate content at the expense of polysaccharides; soft drinks and sweet snacks may constitute a significant part of the carbohydrate intake.
Carbohydrates have differing effects on blood glucose levels and carbohydrate-rich foods can be compared using glycaemic index (GI) ranking. GI values for different foods are calculated by comparing their effect on blood glucose with the effects of a reference food (usually glucose or white bread). Carbohydrates with high GI are digested quickly and absorbed faster so the blood glucose response is fast. Carbohydrates that break down slowly, and result in a slow and sustained release of glucose into the circulation, have a low GI. Low GI foods prolong carbohydrate absorption, attenuate insulin secretion, increase the translocation of the insulin-responsive glucose transporter (GLUT4) to the cell membrane; they also result in more colonic fermentation of carbohydrate and beneficial short-chain fatty acid production High GI foods provide a rapid rise in blood sugar levels and are recommended for post-exercise energy recovery, whereas low GI foods release energy slowly and steadily and increase satiety and are appropriate for diabetics, dieters and endurance athletes. Health benefits of a low GI diet include reduced risk of obesity, diabetes and cardiovascular diseases and lowered incidence of colorectal cancers (Brand-Miller et al., 2009).
Many carbohydrate-rich foods contain indigestible non-starch polysaccharides (NSP or ‘dietary fibre’). Dietary fibre is indigestible carbohydrate. Insoluble fibre promotes the formation of bulkier and softer faecal stools. Soluble fibre slows absorption of glucose and reduces blood cholesterol levels; it is associated with increased insulin sensitivity and decreased incidence of gut diseases. Soluble fibre forms a viscous gel with water and so protects against constipation as makes the faecal stools softer. Foods rich in complex carbohydrates include cereal grains, starchy vegetables, legumes, seeds and wholegrain cereals, all of which contain reasonable proportions (3–15%) of NSP. Most other vegetables, and most fruits, contain small amounts of both starch and NSP and variable amounts of sugars. Most foods that are not highly processed, with the exception of honey and dried fruits, do not contain much sugar, whereas most processed foods contain added sugars, usually sucrose.
Proteins are made up of 20 types of amino acids linked together by peptide bonds. Indispensable or essential amino acids are those that cannot be synthesized from other amino acids in adequate amounts and, therefore, are required in the diet (Table 12.1). There are conditions, in which requirement is high or there is limited ability to interconvert amino acids, that result in an amino acid that can usually be synthesized from an indispensable amino acid being required in the diet. These amino acids are described as being conditionally indispensable, for instance premature babies with immature enzyme function or under conditions of stress may require amino acids that they will be able to synthesize when they are older.
Protein quality depends on the proportion of dietary protein that is absorbed across the gut (digestibility) and the ratio of the essential amino acids in the protein. A protein that is absorbed completely and utilized completely because the indispensable amino acids are in the optimum proportion for synthesis of new proteins is described as a high-quality protein, with a net protein utilization (NPU) value of 1.0 or 100%. Human milk and whole egg have an NPU of 1.0, whereas the overall protein availability in the Western diet is typically 0.7. The NPU of diets dependent on poor-quality proteins, such as those based on cassava (made from tapioca root), can be as low as 0.5.
In the absence of alternative sources of energy, protein can be metabolized as an energy source. Excess protein in the diet will also be used as a metabolic fuel. An adult is usually in nitrogen balance: protein intake is equal to protein breakdown so nitrogen in the diet is equal to excreted levels of nitrogen. Under conditions of growth and protein synthesis, there is a net accumulation of protein, and hence nitrogen, which is described as a state of positive nitrogen balance. States of growth, including pregnancy, result in positive nitrogen balance. Negative nitrogen balance usually indicates tissue breakdown or nutrient deficiency resulting in energy generation from protein sources. Illness and trauma cause negative nitrogen balance, although it also occurs with reduced activity and decreasing muscle mass and during uterine involution (see Chapter 13).
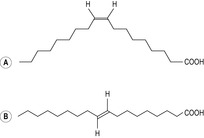
Fat is used for energy requirements. There is also a requirement for essential fatty acids, which cannot be synthesized by the body. These are the precursors of long-chain fatty acids and their metabolic products, prostaglandins and leukotrienes. Fat also provides the vehicle for absorption of fat-soluble vitamins. Most fat is present in the diet as triglycerides; a triglyceride is a glycerol molecule with three fatty acids (Fig. 12.1). There is a range of fatty acids of different chain length and degree of saturation, which is related to the number of double bonds in the fatty acid molecule. Saturated fatty acids have no double bonds, monounsaturated fatty acids have one double bond and polyunsaturated fatty acids have two or more double bonds. The body handles fatty acids differently depending on their length and the degree of saturation (Fig. 12.1C). Fats in foods are formed of triglycerides containing a combination of different fatty acids, but are described by the predominant type. For instance, olive oil is particularly rich in monounsaturated fatty acids (with single double bonds).
Saturated fats are usually solid at room temperature and are usually of animal origin, although coconut and palm oils and cocoa butter have a high level of saturated fatty acids. Saturated fats become rancid very slowly so they store well. Unsaturated fats are usually liquid at room temperature and mostly of plant origin. The C=C double bond is not very stable so it oxidizes easily and the fat becomes rancid. In food processing, unsaturated vegetable oils are hydrogenated (have hydrogen atoms added to saturate the C=C bonds), which makes the fat harder and extends the shelf-life and flavour stability. Unsaturated fatty acids from vegetable and most animal sources naturally adopt a cis configuration, although ruminants produce some trans fatty acids which are thus found in low concentrations in milk and meat from ruminant animals. Positional isomerism, where the fatty acid has the same length and number and position of double bonds, but the hydrogen atoms either lie on the same side of the double bond (cis configuration) or on alternate sides (trans configuration) (Fig. 12.2). Hydrogenation and heating can convert the cis bonds to the trans isomeric forms. Many cellular processes depend on the fluidity of the membrane lipids which depends on the properties of fatty acid chains. Saturated fatty acids are more ordered and rigid. The double bonds of unsaturated fatty acids produce bends in the fatty acid which means that the fatty acids of the membrane pack less tightly together, thus conferring a greater degree of fluidity and flexibility to the cell membrane. Trans fatty acids are straighter and are more like saturated fatty acids in conformation even though they have double bonds. Cholesterol also inserts into the cell membrane bilayer and affects fluidity.
Diets high in saturated fat are associated with an increased incidence of atherosclerosis (damage to arterial blood vessels, causing hardening and plaque formation) and an increase in low-density lipoprotein (LDL) cholesterol levels which is a biomarker for heart disease. Diets higher in polyunsaturated fat are associated with increased high-density lipoprotein (HDL) cholesterol levels and an increased HDL:LDL cholesterol ratio which is associated with more favourable cardiovascular health. Trans fatty acids are implicated in increased risk of myocardial infarction and other cardiovascular problems. HDL levels are also increased by oestrogen (so they are higher in women) and by moderate alcohol intake and exercise.
There are two polyunsaturated fatty acids that are indispensable (‘essential’) in the diet as they cannot be synthesized by the body. These are linoleic acid (18:2, ω-6; chain length of 18 carbons and two double bonds, the first of which is at the carbon atom in the omega position 6 of the chain) and α-linoleic acid (18:3, ω-3; chain length of 18 carbons and three double bonds, the first of which is at the carbon atom in the omega position 3 of the chain). The body can further elongate and desaturate (lengthen and add more double bonds to) these essential fatty acids. However, there are two things to note. First, the fetus has limited ability to elongate and desaturate fatty acids so it is dependent on placental supply for both long-chain polyunsaturated fatty acids (LCPUFA) and the indispensable fatty acids. Second, the enzymes involved in the pathways of elongation and desaturation of the indispensable fatty acids into their longer chain metabolites are competitive. This means that the ratio of ω-6 fatty acids to ω-3 fatty acids is important for optimal development.
Vitamins are organic substances required in small amounts for metabolism, growth and maintenance; they are not synthesized by the body (either at all or in adequate amounts) and so are essential nutrients. Vitamins do not provide sources of energy but act as regulators of metabolic processes. They can be divided into water-soluble (Table 12.2) and fat-soluble vitamins (Table 12.3). The fat-soluble vitamins are more stable than water-soluble vitamins and are stored in the body, so when taken in excess they are more likely to cause toxicity than water-soluble vitamins. As B vitamins function as coenzymes in energy metabolism, requirement for B vitamins increases in parallel with increased energy consumption. Vitamins A, C and E function as antioxidants protecting cells from free-radical damage.
Minerals regulate body function and are essential to good health. They are inorganic and become part of the body structure (Table 12.4). Excessive intake of minerals can be toxic or lead to illness indirectly because of the competitive nature of mineral absorption in the body. For example, excess iron can lead to zinc deficiency and excess zinc can lead to copper deficiency.
The sensitivity of the hypothalamus to environmental influences, such as nutrient availability, was probably of immense importance in promoting pregnancy in seasons when the fetus and infant had optimal chances of survival. Weight loss affects cyclical ovarian function in women. Anorexia nervosa disrupts the hypothalamic–pituitary–ovarian axis (see Chapter 4) and may cause amenorrhoea. Amenorrhoea related to inadequate nutrient intake is often reported in ballet dancers, competitive runners and other athletes (Frisch, 1990). It not only affects the ovulatory cycle but also can result in low levels of oestrogens, which reduce bone density and predispose to osteoporosis. It has been suggested that the menarche depended on women reaching a ‘critical weight’ (Frisch and McArthur, 1974). However, low body weight is not always associated with amenorrhoea. Conversely, dieting, high energy expenditure, nutrient restriction or erratic eating patterns (such as crash dieting and binging) can suppress normal reproductive cycles in women even if their weight stays within a normal range (Coad, 2003).
A minimal level of nutrient intake and fuel metabolism seem to be required to maintain reproductive functions, particularly the pulse generating secretion of gonadotrophin-releasing hormone (GnRH; see Chapter 4). Fluctuations in body fat can also disturb the transport and metabolism of the steroid hormones, which are fat soluble. Nutrient deficiency may itself suppress appetite. Studies in animals suggest optimal pregnancy outcome may depend on long-term nutritional status rather than a period of ‘flushing’ or short-term good-quality diet (Wynn and Wynn, 1991). Although restricted nutrient intake can suppress reproductive function, excess energy intake may also be disruptive. Obesity, in both men (Hammoud et al., 2008) and women (Zain and Norman, 2008), also affects fertility and conception rate. Polycystic ovary syndrome (PCOS), which often causes anovulation (see Chapter 6), is frequently associated with insulin resistance and hyperinsulinaemia even in the absence of obesity (Hirschberg, 2009). However, the symptoms and effects of PCOS on reproduction are more severe with increased body weight. In PCOS, increased obesity disrupts normal production of steroid hormones and affects carbohydrate handling.
A woman’s weight, particularly if it is related to her height, indicates her nutritional status to some degree. Weight loss and nutrient fluctuation caused by self-imposed dieting, affecting reproductive function, may be the cause of infertility in a large proportion of the women seeking fertility treatment. Maternal nutritional status can be assessed by calculation of body mass index (BMI; Box 12.1). BMI is correlated to fat mass (and health prognosis) but there are limitations to its use; BMI tends to overestimate fat mass in individuals who are active and have a high muscle mass and to underestimate fat in individuals who are sedentary. There are also racial differences in the correlation between BMI and fat mass; individuals from African and Polynesian races have less fat per BMI class compared with individuals from Caucasian races, whereas individuals from Asian races tend to have more fat. Whilst it is clear that the time to pregnancy is longer for underweight and overweight women, there is no consensus about the optimal BMI. A BMI of less than 18.5 kg/m2 is not associated with good fertility or pregnancy outcome (Gesink Law et al., 2007). A BMI of greater than 30 kg/m2 in a prepregnant woman is considered a considerable risk factor in the obstetric management of the pregnancy as well as affecting fertility (Lee and Koren, 2010). The higher incidence of obesity in the general population is reflected in the increased number of pregnant obese women who have more pregnancy-related complications such as hypertension, pre-eclampsia and gestational diabetes. Surgery for severe obesity is consequently becoming more commonplace. Bariatric (weight loss) surgery (such as gastric banding or gastric bypass) is an effective treatment for obesity which can restore fertility but as there may be initial nutritional compromise, it is recommended that women leave 2 years after the surgery before conceiving (Shah and Ginsburg, 2010).
Box 12.1
Body mass index (BMI) is used to indicate nutritional status and risk factors associated with obesity. It is a ratio of weight (measured in kilograms) against height (measured in metres squared).
Calculation:
Interpretation:
A healthy shape is considered to be that usually associated with a BMI of 20–25 kg/m2. Waist circumference and waist to height ratios are also used as indicators of healthy shape. A waist circumference of less than 80 cm is considered healthy in women and less than 94 cm in adult men, with waist circumferences above 88 and 102 cm, respectively, indicating risk. A waist:height ratio of less than 0.5 is also considered healthy with a ratio greater than 0.6 indicating risk to health.
Maternal protein intake affects gonadotrophin secretion and ovulatory maturation (Wynn and Wynn, 1991). Diets with abnormally high protein content and also those with very low protein content affect the menstrual cycle and fertility. It is possible that high-protein diets may cause one of the coenzymes involved in protein metabolism to become limiting. It is also suggested that low levels of B vitamins depress pituitary hormone secretion. Both embryonic development, especially early in gestation, and follicular development involve a rapid rate of protein synthesis and cell division, which are associated with a high energy and nutrient requirement. Preconceptual nutrient deficiency may retard development of the follicle and corpus luteum, affecting subsequent embryonic growth, even if the level of deficiency is not adequate to cause infertility. Excess intakes of some nutrients may increase mutation rate. Nutrient deficiency also affects male fertility, by altering DNA synthesis and rates of cell division. Selenium availability may be an important factor in male fertility.
Whether the mother enters pregnancy with high nutritional stores may affect the outcome of the pregnancy. Placental size in humans appears to be governed by genetic growth potential, anoxia and nutrient availability. In sheep, a period of poor nutrition early in gestation increases placental size, presumably as an adaptive mechanism to increase nutrient extraction (McCrabb et al., 1992). Provided this nutrient restriction is transient and the sheep are then returned to richer pasture, the increase in placental size is associated with an increase in lamb birth weight. In humans, an increased placental:fetal weight ratio is associated with poorer outcome and long-term health prognosis (Barker, 1998). However, larger babies have larger placentas but in proportion to their birth weight. Morning sickness may produce a period of poor nutrition in early pregnancy, which could stimulate placental growth (Coad et al., 2002). Provided the woman entered pregnancy with good nutrient stores and the effects on nutrient consumption were limited, nausea in pregnancy could promote placental enlargement and positively affect the fetal growth trajectory. An adequate interpregnancy interval may be important to allow replenishment of maternal stores, especially of vitamins such as folate.
Case study 12.1 looks at an example of assessing nutritional status in pregnancy.
Fiona informs the midwife at the booking appointment that she has a healthy balanced diet.
• How can the midwife assess that this is an accurate statement?
• What observations can help the midwife assess Fiona’s nutritional status?
• Are there any other factors that might affect Fiona’s description of her diet?
• Are there any perceptions of what is a ‘healthy balanced diet’ that may actually be potentially harmful and if so what are they and why should they be avoided?
Food can provide nutrition but it is also the source of a number of maternal infections (Box 12.2). Pregnant women are advised to be particularly careful about food hygiene (Derbyshire, 2010). Although nutrient intake and weight gain are associated with clear effects on birth weight, a number of other factors have been shown to affect fetal size and growth potential (Box 12.3).
Box 12.2
• Caused by: bacterium Listeria monocytogenes
• Possible effects: miscarriage, stillbirth and neonatal death, brain damage, premature delivery, maternal mortality, meconium before 37 weeks gestation
• Sources: soil, soft cheeses, pate, raw seafood, cold meats, poultry, cook-chill food
Note: bacteria can multiply at low temperatures so women are recommended to thoroughly reheat refrigerated left-overs
• Caused by: Salmonella enteric
• Possible effects: maternal high fever, vomiting, diarrhoea and dehydration associated with food poisoning may increase the risk of preterm labour or miscarriage
• Source: raw meat, poultry and eggs, foods made from raw eggs such as mousses and sauces
Note: survives in soft-boiled eggs and mayonnaise, cross-contamination by uncooked foods or utensils is common
• Caused by: Toxoplasma gondii
• Possible effects: congenital mental retardation or blindness, neonatal convulsions, visual and hearing loss, haematological abnormalities, enlarged spleen and liver
• Sources: soil, raw or undercooked meat, cats’ faeces and litter trays, goats’ milk
• Caused by: Campylobacter jejuni and C. coli.
• Possible effects: preterm delivery, intrauterine death
• Sources: undercooked poultry, unpasteurized milk
Box 12.3
• Maternal diet before and during pregnancy
• Maternal size, particularly lean body mass
• Age (younger women tend to gain more weight but pregnancy in adolescents is associated with an increased likelihood of an LBW baby)
• Birth order (first babies tend to be slightly smaller)
• Parity (multigravidae tend to gain less weight)
• Fetal sex (male babies tend to be an average of 150 g heavier)
• Nicotine (both smoking and tobacco chewing are associated with decreased birth weight)
• Alcohol (regular alcohol consumption is associated with lower birth weight)
• Hypoxia (high altitude and chronic maternal anaemia depress birth weight)
Age affects cell proliferation and gamete formation. Some diseases accelerate premature ageing of the germ cells. These include diabetes, parental gene mutation, multiple sclerosis, ulcerative colitis and Crohn’s disease (Wynn and Wynn, 1991). Smoking, drugs, alcohol and radiation all affect cell division; indeed, smoking is probably the most important single factor influencing incidence of LBW babies in developed countries (Chiriboga, 2003). Viruses are mutagenic and have long been associated with abnormal fetal development. Sexually transmitted diseases (STDs) can cause pelvic inflammatory disease, which may affect fertility and pregnancy outcome. Diseases caused by larger organisms, such as syphilis and gonorrhoea, are relatively easy to diagnose and treat. However, STDs caused by smaller microorganisms, such as chlamydia, papilloma, HIV, herpes and mycoplasmas, are difficult to eradicate. STD prevalence is associated with increased mobility of the population.
The nutritional costs of pregnancy can be theoretically calculated by estimating the cost of the new maternal tissues (particularly maternal fat deposition) and the tissues of the conceptus (fetus, placenta, membranes and other tissues) – the ‘capital gains’ – and the metabolic costs of maintaining these growing tissues – the ‘running costs’ (Campbell-Brown and Hytten, 1998). Tissue accrued (based on an assumed body fat retention of 3.8 kg) accounts for about 185 MJ (50000 kcal), and increased metabolism accounts for about 150 MJ (36000 kcal), bringing the total specific cost of pregnancy to about 335 MJ (80000 kcal) (Fig. 12.3). As more studies about nutritional requirements during pregnancy are performed, the recommended daily allowances of energy and other nutrients have progressively decreased. However, recommended allowances are intended to be a standard against which the nutritional status of a population, rather than that of an individual person, can be assessed.
(Reproduced with permission from Hytten, 1991.)
Energy requirements during the pregnancy are highest in the middle (from 10 to 30 weeks) when maternal fat stores are being assimilated. During the last 10 weeks of the pregnancy, the rapid growth of the fetus has a high energy requirement but the rate of maternal fat storage is decreased (and often maternal intake is limited). In effect, the increased nutritional requirements of the pregnancy are spread fairly evenly over the later three-quarters of the pregnancy. The daily increase in energy requirement is calculated to be about 1.2 MJ (300 kcal) over the final three-quarters of pregnancy (Campbell-Brown and Hytten, 1998). This is calculated from the total cost of the pregnancy, estimated to be 335 MJ (80000 kcal) divided by 270 days of pregnancy. The reported energy consumed in pregnancy by women ‘eating to appetite’ (with free access to food) is about 0.8 MJ (200 kcal) extra per day, less than the theoretical expected cost of the pregnancy. Many women, including those in developing countries and the poorer parts of affluent countries, successfully reproduce supported by energy intakes which appear to be well below the recommended levels. Some of this discrepancy is likely to be due to under-reporting of energy intake in pregnancy because of changed eating habits or subject fatigue in research studies.
Some of the additional energy requirements of pregnancy could be met by increased efficiency of maternal metabolism (decreased basal metabolic rate (BMR) or diet-induced thermogenesis (DIT) or the thermic effect of food (TEF)) and decreased activity-related energy expenditure (Forsum and Löf, 2007). However the BMR response to pregnancy is varied (while it is usually stimulated, it may be depressed or unchanged) and DIT (the increase in energy expenditure due to food consumption) probably remains unaltered. Decreased activity-related energy expenditure could make up a considerable proportion of the energy balance but women who are normally sedentary have little flexibility to further reduce their physical activity during pregnancy (Butte and King, 2005). Decreased energy expenditure in the second and third trimesters of pregnancy has been observed in the Five Country Study of pregnant women (Lawrence et al., 1987), which compared women living in Scotland, Holland, the Gambia, the Philippines and Thailand. Leisure activities and the rate at which heavy work was done decreased. Women who have a long history of poor nutrition, for instance those in subsistence farming communities, seem to be able to adapt more to sparing nutrients and economizing to support the cost of the pregnancy. This may be because these women are more physically active and so can decrease their energy expenditure by a greater degree. However many pregnant women living in developing countries are not able to reduce their activity. Physiological adaptation, for instance the lower resistance blood circulation, may alter the efficiency of energy metabolism in pregnancy (Forsum and Löf, 2007).
Dieting or deliberate energy restriction is not appropriate for most pregnant women; it is unlikely to be beneficial and may harm the fetus. Historically, women at risk of developing pre-eclampsia and obese women were recommended to limit their energy intake and weight gain. However, energy restriction has no effect on the development of pre-eclampsia; excessive weight gain is the result, not the cause, of the underlying clinical pathology. Inadequate energy intake, particularly in the first trimester, is associated with an increased incidence of LBW infants and congenital abnormalities (Carmichael et al., 2003). Excessive weight loss and fat mobilization in pregnancy can produce metabolites that can create metabolic stress and are detrimental to fetal development. Maternal health and later lactational capability may be compromised by dietary restriction in pregnancy. In obese women, energy restriction can be associated with lower infant birth weight (Merialdi et al., 2003). However, if obese women are motivated by their pregnant state to follow dietary recommendations and improve their diet, many will lose body fat.
Protein requirements increase in pregnancy, to support maternal tissue synthesis and fetal growth. Metabolic adaptations enhancing the efficiency of protein synthesis are evident from early pregnancy onwards (Duggleby and Jackson, 2002). Low-protein diets are associated with an adverse outcome of pregnancy but low protein intakes are unlikely in affluent developed countries. Protein requirements for the growth of maternal tissues and the growth of the conceptus were calculated to be about 925 g (Campbell-Brown and Hytten, 1998) but have been reassessed to be closer to 500–700 g (Williamson, 2006). The increased protein synthesis, and therefore increased dietary requirement, in late pregnancy is about 6 g/day. In Britain and other developed countries, where the average NPU value is 0.7, this is equivalent to about 8–9 g of additional dietary protein being required to maintain nitrogen balance.
During the pregnancy, there is a fall in blood protein levels from about 70–60 g/L. Much of this fall is due to decreased plasma albumin concentration resulting from haemodilution. Albumin functions as a non-specific carrier of lipophilic substances such as some drugs, hormones, free fatty acids, unconjugated bilirubin and some ions. It has an important role in maintaining the plasma osmotic pressure. The fall in plasma colloid osmotic pressure increases movement of water out of the blood vessels (see Chapter 1), thus increasing lower limb oedema and affecting glomerular filtration rate (GFR). Plasma globulins increase in pregnancy.
Plasma levels of most amino acids fall in pregnancy. The most marked falls are observed in glucogenic amino acids, which can be used to form glucose, then those involved in the urea cycle and then the ketogenic branched-chain amino acids. Amino acids are actively transported across the placenta. The transfer of amino acids across the placenta is only just adequate for fetal protein synthesis so any factor adversely affecting amino acid transport mechanisms has the potential to limit growth. Imbalances in maternal amino acid concentration will be reflected by placental uptake. For instance, women with phenylketonuria (PKU) are advised to resume a low-phenylalanine diet (and take tyrosine supplements) prior to conception as high levels of phenylalanine can harm the fetus, even if the fetus does not have PKU. High phenylalanine levels in pregnancy are associated with fetal IUGR, congenital heart disease, microcephaly and mental retardation. The amino acid methionine is involved in folate metabolism; women who have higher dietary intakes of methionine seem to be at lower risk of delivering a baby with a neural tube defect (NTD; Shoob et al., 2001). Good sources of methionine tend to be foods such as animal proteins that are rich in other amino acids and total protein, iron, zinc and calcium.
The optimum birth weight in humans can be considered to be within the range of birth weights associated with the lowest incidence of perinatal mortality and morbidity, in the range 3500–4500 g (Wynn and Wynn, 1991). Mothers of babies in the optimal birth-weight range tend to eat more protein than women who give birth to babies with lower birth weight. Maternal intakes of B vitamins and some minerals, particularly magnesium, have been found to correlate well with birth weight (Wynn and Wynn, 1991). The main regulator of fetal growth seems to be availability of nutrients, which can affect growth directly by changing the availability of substrates required for growth, or indirectly by altering hormonal control of growth.
The normal protein intake of women in most developed countries, who regularly consume foods such as lean meat and poultry, fish, reduced-fat milk products, wholegrains and legumes as part of a balanced and varied diet, appears to be sufficient to provide the additional requirements of pregnancy. Vegetarian and vegan women must ensure a range of wholegrains and legumes are consumed daily to provide adequate protein. The U.S. Institute of Medicine (2002) recommends an additional 25 g of protein per day for pregnancy in addition to the recommended daily intake (RDI) of 46 g of protein per day (0.80 g/kg/day), a total RDI of 71 g of protein per day for pregnant women (or 1.1 g/kg/day). Women with twin pregnancies are recommended to consume an additional 50 g of protein per day together with an appropriate energy increment to optimize efficient utilization of the protein (Institute of Medicine, 2002). Women with very low energy intakes may be at risk of inadequate protein intake. The use of formulated protein supplements, powders or high-protein formulated beverages should be discouraged because clinical studies suggest they may be potentially harmful to the fetus (Kramer and Kakuma, 2003). Some popular weight-restriction diets have promoted high protein intake. However, the fetus has limited ability to detoxify ammonia and excrete urea, particularly during the vulnerable periods of organogenesis in the first trimester. In experimental animals, high protein intakes and apparent high levels of ammonia have been associated with increased rates of congenital abnormalities.
In pregnancy, plasma lipids alter markedly. Levels of free fatty acids, triacylglycerides, cholesterol, lipoproteins and phospholipids transiently fall early in pregnancy and then rise (Butte, 2000). The changes in handling of lipids are orchestrated by hormonal changes and are associated with changed insulin resistance during the pregnancy (Robinson et al., 1992). The initial low maternal levels of fatty acids reflect maternal fat storage, which is highest early in pregnancy when maternal maintenance costs of pregnancy and fetal growth are relatively low (Campbell-Brown and Hytten, 1998). This appears to anticipate requirements later in pregnancy. In the later stages of pregnancy, when fetal requirements are maximal, maternal nutrient intake could be restricted by lack of availability of food or by restricted capacity for eating and gastrointestinal disturbances. Maternal fat stores, which are 3.5 kg on average, can subsidize a considerable part of the pregnancy. Oxidation of 3.5 kg of fat could theoretically produce 132 MJ (30000 kcal).
During early gestation the fetus depends on placental transfer for fatty acid requirements (Herrera and Amusquivar, 2000). Lipids are transported across the placenta as lipoprotein complexes, classified by their density (Fig. 12.4). Triglyceride levels increase throughout gestation. Placental uptake of triglycerides occurs in the form of very low-density lipoproteins (VLDL). Placental lipase may hydrolyse VLDL, releasing the products for energy metabolism by the fetus (Robinson et al., 1992). The rising level of maternal fatty acids in the third trimester probably reflects mobilization of maternal fat stores. In later gestation, maternal fatty acids are predominantly used for maternal metabolism and ketone body synthesis. The more mature fetus can synthesis fatty acids de novo, using ketone bodies as fuels and lipogenic substrates.
(Reproduced with permission from Saffrey and Stewart, 1997.)
LCPUFA requirements of pregnant women are particularly high, especially in the third trimester when fetal brain and nervous tissue growth is maximal; accretion of DHA into the developing nervous system and fetal brain is high. Arachidonic acid (20:4, ω-6) is essential for neonatal growth and is the precursor for eicosanoids, prostaglandins and leukotrienes, and docosahexaenoic acid (DHA; 22:6, ω-3) has a key role in fetal brain development and visual function. Therefore, fetal demand for indispensable fatty acids (linoleic acid, 18:2, ω-6; and α-linoleic acid, 18:3, ω-3) must be met from either maternal intake or be released from maternal adipose tissue. The placenta transports the indispensable fatty acids and their preformed long-chain derivatives, arachidonic acid and DHA from the maternal circulation to the fetus. Low maternal intake of these indispensable fatty acids is correlated with reduced neonatal growth (Herrera, 2002). Oestrogen increases the conversion of essential fatty acids to long-chain fatty acids.
Consumption of fish, and therefore DHA, in pregnancy is associated with a reduced incidence of pre-eclampsia, LBW and preterm delivery (Makrides, 2009), probably because ω-3 fatty acids from marine sources inhibit ω-6-derived eicosanoids involved in cervical ripening and the initiation of parturition. Women who eat more marine foods also seem less likely to develop pregnancy-induced hypertension (Al et al., 2000). These positive effects of fish consumption on gestational duration and fetal development have generated much interest in requirements of LCPUFA for optimal outcome of pregnancy. However, it is not clear that supplementation with LCPUFA or fish oil might be beneficial as excess intake of LCPUFA can potentially increase the risk of oxidative damage (Herrera, 2002). The higher the content of PUFAs in the diet, the more likely damaging free radicals will be formed which are potentially toxic and can reduce antioxidant capacity.
Western diets are relatively rich in ω-6 fatty acids but poor in ω-3 fatty acids and intake of preformed DHA is low, so the supply of DHA to the fetus may be compromised. The ratio of ω-6 fatty acids to ω-3 fatty acids is estimated to be significantly higher than it was in the Neolithic era when the big brain of modern man evolved. Obese women with insulin resistance and thin women with little body fat are likely to be even more dependent on dietary DHA. Eating fish in pregnancy would increase DHA intake but much of the advice about fish liver oils containing vitamin A and not consuming an excess of fish in case of heavy metal contamination of fish has led pregnant women to avoid fish totally in pregnancy rather than increase their intake because they are pregnant.
A high fat intake is not recommended in pregnancy as there is an association between increased fat intake and the development of glucose abnormalities (Saldana et al., 2004). Ketonaemia appears to have a negative effect on fetal development and later intellectual performance (Rizzo et al., 1991). High-fat, low-carbohydrate diets are not optimal for pregnancy; it is suggested that a diet <30% fat and >50% carbohydrate reduces the risk of glucose intolerance and gestational diabetes.
Adequate carbohydrate intake is important in pregnancy to ensure adequate glucose for maternal brain metabolism and transfer to the fetus but normal diets are usually rich in carbohydrates so there is no changed recommendation for pregnancy. Metabolism of carbohydrates and lipids alters, under hormonal influence, throughout pregnancy to ensure that the fetus receives a continuous supply of nutrients despite maternal intake being intermittent (Butte, 2000). Maternal glucose concentration is maintained at a significantly higher level in later pregnancy by increased hepatic glucose production in order to meet the increasing requirements of the placenta and fetus. The developing fetus utilizes glucose as its primary energy-producing substrate but it can also metabolize maternally derived ketoacids.
Get Clinical Tree app for offline access