Chapter 38 Managing HIV infection in children and adolescents
Epidemiology
The epidemiology of HIV disease in children in developed countries has changed substantially. These changes have been driven by the evolving epidemiology of HIV infection in women, the ability to prevent mother-to-child transmission, the dramatic increases in survival for children treated with combination antiretroviral therapy (CART) [1–3], and the evolution of drug resistance. Women constitute a significant proportion of AIDS cases and new HIV infections. Women account for 27% of new HIV infections in the United States [4]; this has remained stable over the last 5 years. However, women account for 25% of the estimated 1 million people living with HIV in the United States, suggesting that 250,000 women are infected [4, 5]. In 2005, the Centers for Disease Control (CDC) estimated that 6,000–7,000 infants were born to HIV-infected women each year in the USA [6]; however, only 141 new perinatal infections were reported in 2008 [5].
Since 1992, the number of children younger than 13 years diagnosed with AIDS in developed countries has decreased dramatically, representing one of the remarkable successes in the fight against HIV. In the USA, the estimated number of children younger than 13 diagnosed with AIDS decreased from 952 in 1992, to 34 in 2008 [5]. This likely represents improvements in clinical care and survival, but this has led to a larger population of surviving children. By the end of 2008, an estimated 908 children were living with AIDS in the USA and its territories, and an additional 2,919 children were reported to be living with HIV infection (not AIDS) from the 37 states with name-based reporting [5]. No accurate data are available regarding the number of HIV-infected immigrants, refugees, and adopted children living in the United States. However, foreign-born children may represent a significant portion of HIV-infected children entering care, and they may face unique challenges.
Natural History
Timing of infection
HIV progresses more rapidly in children with perinatal infection than among children infected at an older age or among adults. It has long been recognized that there was a bimodal distribution of clinical progression following perinatal infection [7–9]. About 20% of children had early onset of symptoms before the advent of effective therapy. These children have a rapid downhill course in the first 12 months of life, marked by rapid decline in CD4 count, and development of category C disease, often with pneumonia due to Pneumocystis jiroveci (formerly P. carinii pneumonia), or death.
There appear to be a number of predictors of rapid progression, including severe maternal disease [10], evidence of in utero transmission (positive PCR at birth), early hepatosplenomegaly [11], and higher viral loads after 1 month of life [12, 13]. CD4 and CD8 counts below the fifth percentile in infancy were associated with rapid progression among babies infected in utero in one study [14], perhaps reflecting early destruction of the thymus.
The natural history of HIV among adolescents who have acquired infection through adult behaviors generally parallels adults. However, younger age at infection for those with non-perinatally acquired HIV is associated with significantly slower progression in the absence of antiretroviral therapy (ART) [15, 16].
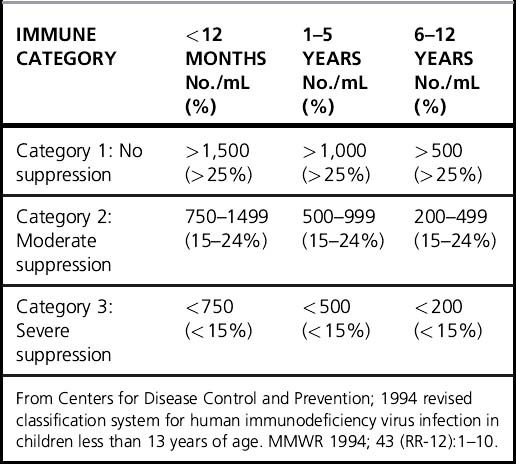
Declining CD4 count and CD4% are the hallmarks of HIV disease progression in children. CD4 count normally declines with age in young children, making interpretation somewhat difficult. CD4% is less age-dependent and is also useful in disease staging (Table 38.1) [17]. The revised CDC, PENTA, and WHO classifications use both immunologic status and clinical status for staging. Growth failure is a sensitive indicator of disease activity, and improved growth is a marker of successful ART.
Predicting progression
Quantitative measurement of plasma viral RNA revolutionized the management of HIV in adults; similar data in children required a few more years to accumulate [13, 18, 19]. The kinetics of plasma HIV RNA in children differs from adults in several ways. First, children tend to have higher viral loads, with median peak values between 100,000 and 1,000,000 copies. Second, after primary infection, the viral load slowly declines during the first year of life, in contrast to the rapid 2–3 log drop in adults. Third, although viral load is consistently associated with prognosis, it has been difficult to establish specific levels that are sensitive and specific for high risk [20]. These differences may reflect a greater number of target cells and a limited ability to mount an immune response by the immature immune system. Children infected in utero tend to have modest viral loads at birth, but the peak value at 1–2 months is higher than those with presumed intrapartum infection.
A pivotal meta-analysis of survival data on 3,941 European and American children with HIV infection in the pre-HAART era demonstrated that CD4% and viral load were independent predictors of progression to AIDS or death over the next 12 months. CD4% was the strongest short-term predictor (Fig. 38.1) [20]. The risk of progression at a given CD4 level of viral load varied by age. Importantly, among children < 12 months, the risk of progression remained moderately elevated even when the CD4% was high or the viral load was low. A recent analysis of the same cohorts concluded that CD4 count may be more useful than CD4% in determining treatment initiation, particularly if only one has crossed a threshold [17].
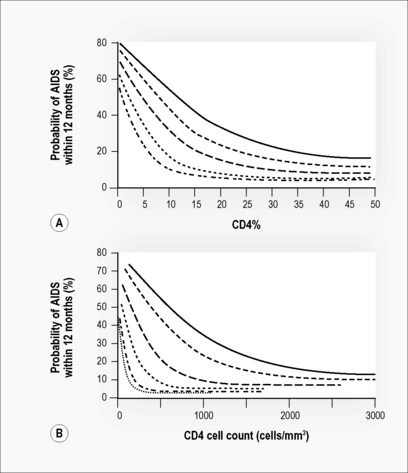
Figure 38.1 Probability of developing AIDS in the next 12 months by age group.
(A) By CD4%; age groups (top to bottom) = 6 months, 1 year, 2 years, 5 years, 10 years.
Adapted from Dunn [20]. (B) By CD4 count; age groups = 6 months, 1 year, 2 years, 3 years, 4 years, 10 years. Adapted from HIV Paediatric Prognostic Markers Collaborative Study [35].
With the advent of three-drug combination ART for children, survival has increased dramatically. In a cohort of 1,000 children in the UK, there was an 80% decline in mortality and a 50% decline in progression to AIDS between 1997 and 2002, along with a 80% decline in hospital admission rates between 1996 and 2002 [21].
Early Diagnosis and Management of the Exposed Infant
Diagnosis of HIV infection
Viral culture is no longer used for diagnosis of HIV in infants. Currently, either detection of DNA or RNA by PCR is the test of choice. HIV DNA PCR is only moderately sensitive in the first 48 hours of life (38%; 90% confidence interval [CI], 29–46%). Sensitivity rises rapidly during the second week; 93% of infected children (90% CI, 76–97%) were PCR positive by 14 days of age. Quantitative RNA PCR is at least as sensitive and specific as DNA PCR, and offers the advantages of using smaller blood volumes, providing important prognostic data [22, 23], and being more sensitive for detecting non-clade B strains [24]. False positives can occur; levels < 5,000 copies/mL should be considered suspect. Measurement of p24 antigen, either conventionally or with immune dissociation, is not recommended for the diagnosis of neonatal HIV infection because it is less sensitive and specific than PCR.
Monitoring in the HIV-exposed or HIV-infected infant
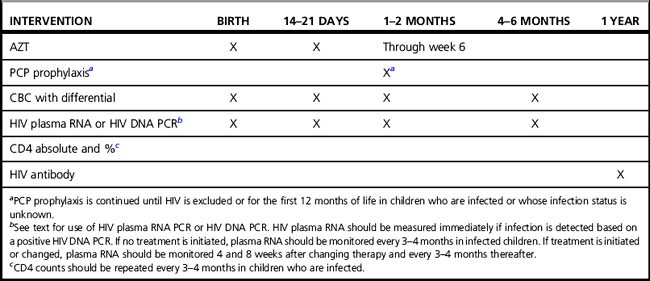
Infants born to HIV-infected women should receive oral AZT during the first 6 weeks of life, based on the PACTG 076 protocol. Myelosuppression is common with both AZT and trimethoprim-sulfamethoxazole, and the complete blood count should be monitored. Plasma viral RNA and CD4 count and percentage should be monitored immediately once the diagnosis of HIV is established, and followed every 3–4 months. When starting ART, plasma viral RNA and CD4 count should be measured at baseline, after 1 and 2 months, and every 3–4 months thereafter. A suggested monitoring scheme is shown in (Table 38.2).
Vaccination
Timely vaccination is important for HIV-infected children. Guidelines are available [25]. Inactivated vaccines (hepatitis B, Haemophilus influenzae type B, diphtheria-tetanus-pertussis, IPV) are given according to the schedule recommended for all children. Measles, mumps, and rubella (MMR) and varicella vaccines are live-attenuated, which pose a theoretical risk to severely immunocompromised children. The vaccines should not be given to those with CD4% less than 15%. HIV-infected children without immunosuppression should receive their first dose of MMR as soon as possible after the first birthday. The second dose does not need to be delayed until school entry; it can be given as soon as 1 month after the first dose. Annual immunization against influenza is recommended for all HIV-infected children. Initially, two doses are given, separated by at least 1 month.
Since infections with encapsulated bacteria are prominent among HIV-infected children, the potential benefit of pneumococcal vaccine is large. Unfortunately, children < 2 years old respond poorly to polysaccharide vaccines. In February 2010, a new 13-valent pneumococcal conjugate vaccine (PCV-13) was approved by the FDA, which offers expanded coverage of the serotypes causing the majority of invasive pneumococcal disease (IPD) in children. PCV-13 is recommended for all children younger than 72 months with an underlying medical condition, including HIV. In addition, children aged 6–18 years may receive one dose of PCV-13. A single dose of PCV-13 should be given to children younger than 6 years who were previously vaccinated with PCV-7. After completion of the PCV-13 series, the 23-valent pneumococcal vaccine (PPSV23) should also be given after 24 months of age. Re-vaccination should be offered 5 years after the first dose of PPSV23 [26].
Vaccination is also important for HIV-infected adolescents. They should receive pneumococcal and annual influenza vaccinations. Their immunization status to hepatitis A, hepatitis B, and measles should be reviewed and updated. Meningococcal conjugate vaccine and tetanus diphtheria acellular pertussis (Tdap) are recommended for all adolescents. Human papillomavirus vaccine is licensed for use in both females and males; however, no specific recommendations exist regarding its use in HIV-infected children and adolescents [4, 27]. Given the increased risk of HPV-related disease among HIV-infected persons, HPV vaccine (HPV4) should be strongly considered for both female and male HIV-infected adolescents.
Antiviral Therapy
Principles of therapy
It is important, however, to appreciate ways in which children differ from adults. The majority of HIV-infected children are infected around the time of delivery, and therapy can potentially be started during primary infection. Theoretically, this offers children an advantage that is rare in adults. Intact thymic architecture offers the potential for greater immune reconstitution, and in one study, thymic volume on CT scan correlated with completeness of immune reconstitution [28].
When to start
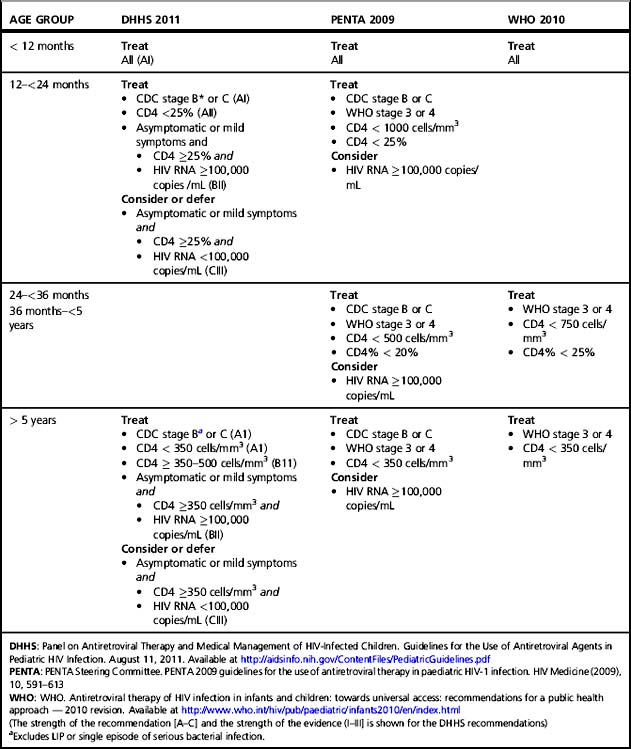
Recommendations on starting therapy and preferred regimens have been formulated by the Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children in the US and the Paediatric European Network for Treatment of AIDS (PENTA) (Table 38.3) [24, 29]. These guidelines are generally concordant but have subtle differences. The decision to start therapy balances the probability of developing severe clinical disease in the near term and the risk of irreversible damage to the immune system or developing organs with the known difficulties of maintaining suppression in children, short-term side effects, the risk of developing drug resistance, and the possibility of running out of effective agents. In addition, uncertainty remains about the importance and frequency of long-term toxicities in children, including abnormalities of lipid, glucose, and bone metabolism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree