Consultant in Paediatric Respiratory Medicine, Nottingham Children’s Hospital
- understand the range of pathogens causing lung infection in children
- be aware of strategies to prevent serious lung infection in children and to prevent cross-infection in the hospital setting
- understand the diagnostic difficulties which may arise
- be familiar with the emergency management of paediatric lung infection
- be familiar with the specific antimicrobial therapies available and ways of improving effectiveness through increased adherence.
Introduction
Respiratory infections in children are common. One-third of all preschool children will be seen in primary care at least once a year because they are coughing (Hay et al. 2005). Respiratory infections are one of the most common reasons for children to be admitted to hospital; respiratory syncytial virus (RSV) infection alone is estimated to account for 20% of hospital admissions in preschool children in the US (Hall et al. 2009). The cost to the UK National Health Service of treating acute cough in preschool children has been estimated at over £30 million per year (Hollinghurst et al. 2008).
Respiratory symptoms (such as poor feeding, cough and difficulty breathing) may indicate a self-limiting upper respiratory infection or the early stages of a severe lower respiratory infection (such as bronchiolitis, croup or pneumonia). Careful nursing observations will ensure effective triage and assessment, as well as guiding the supportive treatment which is the cornerstone of care.
Bronchiolitis
Acute bronchiolitis is one of the most common causes of hospital admission in children in the UK, during the months of November through to the end of February. In other parts of the world, different seasonal peaks occur and, in many tropical countries, the peak in admissions corresponds with the rainy season.
Bronchiolitis is most commonly caused by RSV but other important causes are human metapneumovirus, parainfluenza virus type 3, influenza and adenovirus. The clinical syndrome is the same, irrespective of the virus responsible, with the exception of some forms of adenovirus which have the capacity to cause severe pneumonia and a form of permanent lung damage known as obliterative bronchiolitis. Infants have smaller airways than older children and these are more likely to become blocked (leading to air trapping) when inflammation, due to virus infection, produces inflammatory secretions and oedema. A 1 mm rim of oedema in an airway of 4 mm in diameter will increase airways resistance 16-fold. Much of the inflammation in bronchiolitis is caused by neutrophils and an excessive response by the infant’s immune system (McNamara and Smyth 2002).
Bronchiolitis is primarily a condition of the first year of life. It is a clinical syndrome, characterised by coryza, cough and a chest which appears hyperinflated (air ‘trapped’ in the chest). When a stethoscope is applied, crackles are heard at the lung bases. In North America, the term bronchiolitis may be applied to young children with episodic (viral) wheeze.
In most cases the infant’s mother will report poor feeding. In the early stages of infection, this may be due to coryza, causing nasal obstruction. As infection progresses over several days, poor feeding may be related to tachypnoea. Where this is severe, infants are at risk of aspiration. Infants under 6 weeks of age with RSV infection are at risk of central apnoea.
There is no active immunisation against RSV infection. Infants with chronic lung disease of prematurity who are receiving home oxygen, those with some forms of congenital heart disease and severe immune deficiency are at greater risk of severe bronchiolitis. Passive immunisation with palivisumab (a monoclonal antibody against RSV) should be offered to these children (JCVI 2004). This is expensive and needs to be administered at monthly intervals usually in five doses over the winter period.
Viral diagnostic tests may help with measures to prevent cross-infection in hospital, e.g. nursing infants with RSV infection in cubicles or cohorting larger groups with RSV infection in ‘bronchiolitis bays’ (SIGN 2006). Each institution will have its own infection control policy, which should be followed. Nasopharyngeal aspirates can be analysed by direct immunofluorescence but increasingly polymerase chain reaction (PCR, see Glossary) methods are used. A chest x-ray is rarely helpful, unless a complication such as aspiration is suspected.
The treatment of viral bronchiolitis is supportive. Babies who are feeding poorly should have nasogastric feeds (SIGN 2006). However, if the infant is very tachypnoeic (>60 breaths per minute), consider nil by mouth and intravenous fluids for a short period to avoid aspiration of feeds. Supplementary oxygen should be administered if the oxygen saturation is consistently <92%. This is best given through nasal cannulae. A small proportion of infants, often those with a known risk factor for severe disease (see above), may require ventilatory support. There is as yet no evidence that the use of nasal continuous positive airway pressure (nasal CPAP), in a deteriorating infant, will avoid intubation and mechanical ventilation (Palanivel and Anjay 2009). Many infants require a ‘trickle’ of oxygen to maintain normal oxygen saturations levels for several days after their respiratory symptoms have settled.
A borderline saturation in air, in an otherwise well baby, is not a reason to keep them in hospital. Simple nursing measures, such as gentle suction of nasal secretions, may also be helpful. One specific therapy has been shown to be of benefit in recent years – nebulised hypertonic saline (Zhang et al. 2008). This is administered as a 3% solution (usually 4 mL nebulised very 8 h) and in randomised controlled trials has improved clinical score and shortened hospital stay by approximately 24 h.
Ribavirin is an antiviral agent which is effective against RSV and may be given in nebulised form. It can cause genetic abnormalities in pregnancy, it is also difficult to administer and there is little evidence of benefit (Ventre and Randolph 2007). Bronchodilators, steroids and antibiotics are of no benefit in most infants with bronchiolitis and should not be used (although they frequently are) (Calogero, Sly 2007; Gadomski, Bhasale 2006; Spurling et al. 2007). Young children who are admitted with acute bronchiolitis may go on to have recurrent episodes of wheezing in the coming months. There is no effective preventive treatment for this complication (Blom et al. 2007). Even at 10 years of age, children who were admitted with bronchiolitis in infancy will wheeze more frequently than matched controls (Noble et al. 1997).
Pneumonia
Pneumonia is the most common cause of death in children under 5 years worldwide, accounting for 2 million deaths (19% of all deaths) (Bryce et al. 2005). In 2000, 42 countries accounted for 90% of deaths from pneumonia and over half the children in these countries failed to get the antibiotic they needed (WHO 2005). In the developed world there are few deaths and the incidence of pneumonia in children under 5 is around 36 cases per 1000 children per year, just under half requiring admission to hospital (Jokinen et al. 1993).
In the UK, infection with some of the organisms which can cause pneumonia may be prevented through the primary immunisation schedule. These include pneumococcus and pertussis (UK Immunisation Schedule 2010). Children in at-risk groups should also be given specific immunisations, e.g. influenza immunisation for children with chronic respiratory disease, such as cystic fibrosis, and 25-valent pneumococcal vaccine for children with sickle cell disease (Department of Health 2010).
Infants with pneumonia may present with non-specific symptoms such as poor feeding, lethargy and fever. There may be specific respiratory signs such as grunting and tachypnoea. However, in children under a year, the presentation may be one of ‘sepsis’ with a respiratory cause only apparent once an infection ‘screen’ has been undertaken and antibiotics started. Even in older children, symptoms may be misleading – abdominal pain is seen as well as chest pain and cough may be a late feature. In young infants, respiratory examination may reveal tachypnoea and chest indrawing. In older children classic physical signs such as a dull sound when the chest is percussed (tapped) and bronchial (harsh) breathing are more likely to be present and may allow the affected lung (and lobe) to be determined (Smyth 2001).
In younger children, viral infection (such as RSV) is the most common cause of pneumonia. Bacterial organisms are more common in older children, most commonly Streptococcus pneumoniae (‘the pneumococcus’), followed by Haemophilus influenzae, Mycoplasma pneumoniae and Chlamydia pneumoniae. The pneumococcus is transmitted through droplet spread (close prolonged contact) and some forms can live in the nasopharynx without causing symptoms. Young children are at greater risk of pneumococcal infection than adults, particularly children with poor splenic function (e.g. sickle cell disease) or immune deficiency. Staphylococcus aureus is an uncommon cause of pneumonia. Children appear much sicker, and may develop complications such as pneumatocoele (thin-walled cavities on chest x-ray). If Staph. aureus is suspected, intravenous flucloxacillin should be given. As with bronchiolitis, a nasopharyngeal aspirate may help to identify the causative virus in infants.
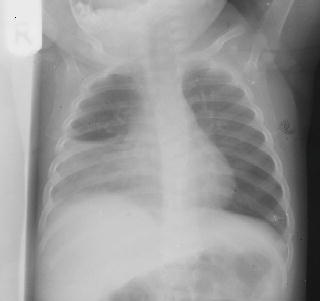
Diagnosis of bacterial infection is more difficult. Children admitted to hospital should have blood sent for culture (though the test is insensitive), and serum sent immediately and at 6-week follow-up looking for antibodies to atypical organisms such as Mycoplasma and Chlamydia. Where a firm clinical diagnosis has been made, a chest x-ray is not necessary (Figure 8.1) (BTS 2002).
Oxygen should be administered to all children with an oxygen saturation <92%. Some children will have become dehydrated and will need nasogastric or intravenous fluids. Most children with pneumonia can be treated with oral antibiotics (Atkinson et al. 2007). Exceptions include those with very low oxygen saturation (<85%), children who are shocked and those who are immune deficient (Atkinson et al. 2007). Oral amoxicillin is a good first-line treatment (or intravenous benzylpenicillin if oral antibiotics are contraindicated). If infection with Mycoplasma or Chlamydia is suspected, then a macrolide antibiotic such as clarithromycin should be given. Children who are drinking adequately and who no longer require oxygen can complete their treatment at home.
Empyema
Uncommonly, children with pneumonia develop a persisting fever and worsening dyspnoea. Percussion of the chest reveals dullness on one side, there may be a small patch of bronchial breathing above the dull area and the spine may curve to avoid expanding the chest on the affected side (scoliosis). A chest x-ray will reveal a dense white shadow on the affected side indicating fluid between the lung (which is lined by the visceral pleura) and the chest wall (parietal pleura). This is called a pleural effusion and it occurs when inflammation of the lung spreads to involve the pleura and fibrin ‘gums up’ the pleural lymphatics. Initially the fluid may be clear (uncomplicated parapneumonic effusion) but in many cases the pleural effusion itself becomes infected (complicated parapneumonic effusion or empyema). In some cases the effusion develops without evidence of prior pneumonia and a child with pneumonia can develop a large pleural effusion over the space of a few days. Empyema has become more common in the UK in recent years (Rees et al. 1997).
Mycoplasma and Chlamydia usually cause only small effusions and the most common organism in empyema is the pneumococcus, though Staph. aureus, other streptococci and gram-negative organisms can be responsible. Children with suspected empyema should have a chest x-ray and ultrasound scan of the chest (Balfour-Lynn et al. 2005).The latter will show if pleural thickening and fibrinous loculations (pockets) are present, which may make it difficult for fluid to drain and antibiotics to penetrate.
All children should have pleural fluid sent for culture. Prolonged antibiotic treatment (often for several weeks) may be needed. Initially, intravenous benzylpenicillin (with flucloxacillin if Staph. aureus is suspected) should be given and antibiotics amended if and when culture results become available (Balfour-Lynn et al. 2005).
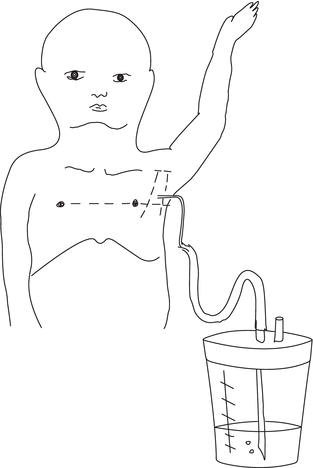
In most cases empyema can be effectively treated with a small (10–12 Fr) chest drain (inserted using the Seldinger technique), though occasionally a larger drain may be inserted under anaesthesia. The drain is connected to an underwater seal (Figure 8.2) and low-grade suction (-20 cm water) may be applied (see below). The site of insertion is determined by chest ultrasound, though larger drains should be inserted in the ‘triangle of safety’ bounded by pectoralis major medially, latissimus dorsi laterally and the internipple line below (see Figure 8.2).
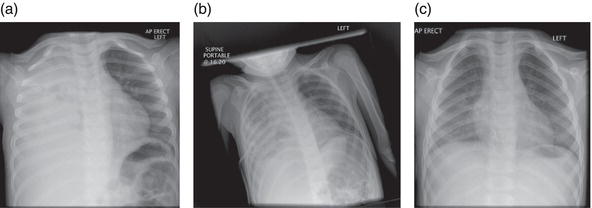
A large volume of purulent fluid may drain initially. Once rapid drainage has ceased, urokinase should be instilled into the drain. A commonly used approach cited by Thomson et al. (2002) is to instil 40,000 units of urokinase in 40 mL of saline which is left to dwell for 4 h with the drain clamped and then placed under low-grade suction for 8 h. Six × 12-hcycles are given over 3 days. If ultrasound shows thick pleural ‘peel’ or fibrous loculations, or initial management is unsuccessful, then surgical decortication may be necessary (Eastham et al. 2004). Following discharge, a repeat chest x-ray should be performed after at least 6 weeks have elapsed. Figure 8.3 shows a sequence of x-rays in a child with empyema, showing the effects of drain insertion and appearance at follow-up.
Figure 8.3 A series of chest x-rays of a 5-year-old boy who presented with a 1-week history of fever and shortness of breath, caused by right-sided empyema. Note the scoliosis with the thoracic spine flexed to the patient’s right in (a) and (b). After drain placement, the patient received 3 days of urokinase instillation as described in the text. (a) Initial presentation. (b) After placement of a right-sided narrow-gauge chest drain. (c) At follow-up 6 weeks later.
Infectious and non-infectious causes of acute stridor in children
The most common cause of acute stridor in children is viral croup. Box 8.1 shows the differential diagnosis of acute stridor in children. For the sake of completeness, two non-infectious causes (inhaled foreign body and anaphylaxis) are also included.
Croup
Viral croup or laryngotracheobronchitis occurs most frequently in spring and autumn and affects preschool children. The most common organisms are parainfluenza virus types 1 and 2, though other viruses may be responsible – RSV, rhinovirus and (in the unimmunised) measles. Partial airways obstruction is caused by inflammation of the vocal cords and trachea. Clinical features include a characteristic barking cough, hoarse voice and inspiratory stridor. There is often a prodromal viral illness. The diagnosis is clinical – blood tests and x-rays are unhelpful.
Management depends on the severity of respiratory distress and any hypoxaemia but should initially follow the airway, breathing, circulation (ABC) approach. Toddlers with croup are best left sitting on their mother’s lap and will automatically adopt the best position to maintain their own airway. Oxygen should be held gently to the face, if the oxygen saturation is <92%. Croup scores are frequently used in clinical trials but not in routine clinical practice. The Westley score is reliable and has been validated (Westley et al. 1978). The essential components of this describe the severity of croup using a scoring system, which enables an assessment of the presenting symptoms for a child with croup, which is summarized in Table 8.1.
Steroid treatment is proven to be effective and can be administered as nebulised budesonide (relatively expensive) or oral dexamethasone (Russell et al. 2004). Both treatments work quickly (within 6 h) and last for up to 12 h. Steroid treatment reduces the duration of hospital stay by an average of 12 h and reduces readmissions. There is no difference in the (small) number of children needing intubation and intensive care, with steroid use. Budesonide is administered as a fixed dose of 2 mg and dexamethasone at a dose of 0.3 mg/kg. In either case, a single dose should be administered and the effect reviewed after several hours. Nebulised adrenaline (1–5 mL of 1/1000) may be used as a temporising measure in children with croup, while waiting for expert anaesthetic support to arrive.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree