Chapter 34 Management of herpesvirus infections (cytomegalovirus, herpes simplex virus, and varicella-zoster virus)
Cytomegalovirus
General comments
With the advent of highly active antiretroviral therapy (HAART), there has been a marked decline in cytomegalovirus (CMV) disease except in the developing world, where up to 25% of AIDS patients may develop end-organ CMV disease. Even where antiretroviral therapy (ART) is available, the syndromes discussed below still occur in the early months of therapy, because the CD4 lymphocytes require weeks to months to become fully functional [1]. CMV disease is also seen in patients who have eluded medical care and in those who fail or are intolerant of ART [2].
Serologic evidence of infection with CMV is extremely common in HIV-infected patients, and the virus can cause several clinical illnesses, including chorioretinitis, esophagitis, colitis, pneumonia, and several neurologic disorders in patients with severe immunosuppression. Even patients with blood, urine, or tissue cultures positive for CMV may not develop clinical illness related to the infection. However, in patients with advanced AIDS (CD4 counts of < 50 cells/mm3), the risk of developing CMV disease and death is directly related to the quantity of CMV nucleic acid in plasma. In a study of over 600 advanced AIDS patients, each log10 increase in baseline CMV DNA was associated with an approximate three-fold increase in CMV disease and a twofold increase in mortality at 1 year [3].
Diagnosis of CMV disease may require tissue biopsy with histologic evidence of viral inclusions, antigen or nucleic acid in tissue. This section reviews the most common clinical manifestations of CMV and their management (Table 34.1).
Table 34.1 Treatment of cytomegalovirus (CMV) infection in AIDS patients
| Preferred therapy | Alternative therapy | |
|---|---|---|
| CMV retinitisa,b,c | ||
| Sight-threatening lesions (induction therapy) | Ganciclovir implant + valganciclovir or valganciclovir | GCV IV, Foscarnet IV, Cidofovir IV |
| Peripheral lesions (induction therapy) | Valganciclovir | Same as above |
| Maintenance therapy | Valganciclovir | Same as above |
| Relapsing and/or GCV resistant | Re-induction with ganciclovir/valganciclovir +/− ganciclovir implant; foscarnet IV +/− ganciclovir/valganciclovir | Cidofovir (if only UL97 mutation); fomivirsen |
| CMV gastrointestinal disease | Ganciclovir IV Foscarnet IV or Valganciclovir (if absorbing) | |
| CMV neurological disease | Ganciclovir IV + foscarnet IV |
a If not already begun, ART should be initiated concurrent with anti-CMV therapy, except possibly when treating CNS disease.
b For retinitis, anti-CMV therapy should be continued until CD4 count has exceeded 100–150 cells/mm3 for 3-6 months and retinitis is inactive. If anti-CMV therapy is discontinued, regular eye exams should be performed every three months.
c Early relapses of CMV retinitis in patients treated systemically are usually due to inadequate drug penetration; re-induction with the same drug is often effective. Drug resistance may occur in patients treated for > 3 months. Therapy of these patients may be guided by antiviral susceptibility testing.
Chorioretinitis
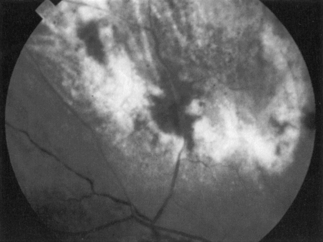
Ocular disease caused by CMV occurs only in patients with severe immunodeficiency and was especially common in patients with AIDS prior to the advent of HAART. Clinical evidence of CMV retinitis (Fig. 34.1) occurred in as many as 40% of AIDS patients, and autopsy series revealed that CMV retinitis was present in up to 30% of patients. With the routine use of prophylaxis against Pneumocystis pneumonia, retinitis became a common presenting manifestation of AIDS, but it occurred more often months to years after the diagnosis of AIDS had been established. The incidence of CMV disease is currently low, primarily because of the efficacy of ART in preventing severe immunosuppression.
Decreased visual acuity, the presence of floaters, or unilateral visual field loss are the most common presenting complaints. Ophthalmologic examination typically reveals large creamy to yellowish white granular areas with perivascular exudates and hemorrhages (Fig. 34.1). These lesions initially occur more often at the periphery of the fundus and, if left untreated, progress centrally within 2–3 weeks. Retinitis usually begins unilaterally, but progression to bilateral involvement is common because of associated viremia. Systemic CMV infection involving other viscera may be present.
Virtually all patients with CMV retinitis have CD4 counts of < 50 cells/mm3. Ophthalmologic screening of patients with pupillary dilation, and ideally, indirect ophthalmoscopy, may be valuable when cell counts decline to this level. It is also important to ask about visual abnormalities, especially increased floaters or visual field defects, and to examine the fundus carefully when there are visual complaints. Patients with confirmed CMV chorioretinitis should be treated with ganciclovir, valganciclovir, foscarnet, or cidofovir [4–6]. These agents are equally effective in the treatment of CMV retinitis [7]. The toxic effects of these agents vary widely but the usual drug of choice is ganciclovir/valganciclovir. In resource-poor settings, intravitreal treatment may be employed.
Nervous system
Encephalitis with dementia and ventriculoencephalitis
CMV encephalitis (CMVE) with dementia and ventriculoencephalitis are the two syndromes of CMVE described in AIDS patients. CMVE with dementia, the more common of the two syndromes, is well described neuropathologically [8] as a multifocal, scattered micronodular encephalitis that resembles HIV encephalitis, the cause of HIV-associated dementia (HIVD) [9]. CMV ventriculoencephalitis is a late and terminal event with acute onset of encephalitis often associated with cranial nerve involvement and nystagmus. The significance of differentiating CMVE and HIVD lies in the different drugs available for treatment.
CMVE is seen more commonly among men who have sex with men (MSM), which may reflect the increased CMV seroprevalence in that population [10]. CMVE always occurs in patients with CD4 counts of < 100 cells/mm3 and should be suspected in patients presenting with a subacute encephalopathy who have had AIDS for more than 1 year. Clinicians should suspect a diagnosis of CMVE in patients who have a history of systemic CMV infection, especially those with CMV retinitis who develop encephalopathic features and change in mental status.
Patients with dementia caused by CMVE usually have a more acute onset and rapid progression than patients with HIVD. The encephalopathic symptoms include delirium and confusion, lethargy and somnolence, apathy and withdrawal, personality changes, and focal neurologic signs with cranial nerve involvement. During the course of illness, recurrent fever episodes may occur that may be attributed to other opportunistic infections (e.g. Mycobacterium avium complex). Psychomotor slowing, primitive reflexes, and peripheral neuropathy may also be seen in CMVE. Distal sensory polyneuropathy usually antedates the onset of CMVE [11].
The course of encephalopathic illness in both CMVE and HIVD includes progressive worsening in mental status until death. The median survival of CMVE patients is significantly shorter (weeks) compared with that of HIVD patients (months). Autopsies reveal a range of neuropathology, including ependymal and subependymal necrosis, areas of demyelination, and microglial nodules that are more frequently encountered than typical nuclear and cytoplasmic CMV inclusions [12]. Neuropathologic evaluations of CMVE and HIV co-infection of single cells suggests that CMV and HIV increase each other’s replication in the brain [13].
It is difficult to make a definitive diagnosis of CMVE, and laboratory investigations are not helpful in distinguishing CMV from HIVD. Electrolyte abnormality, especially hyponatremia, is more commonly present in CMVE patients [14]. There are insufficient data to determine whether CMV antigen or DNA is regularly detected in CSF. Conversely, detection of CMV DNA may only reflect latent, but not active, CMV diseases. MRI scans showing meningeal enhancement consistent with ventriculitis and periventricular enhancement are helpful in differentiating CMVE from HIVD. However, periventricular enhancement may also be seen in lymphoma, toxoplasmosis, and pyogenic brain abscesses. Progressive ventriculomegaly, if seen in serial computed tomography scans, is highly suspicious for CMVE [15, 16].
Mononeuritis multiplex
This is the least common of all the neurologic syndromes attributed to CMV. Clinical characteristics of CMV mononeuritis are more varied than polyradiculopathy/myelitis. Patients may present with multifocal, patchy and/or asymmetrical sensory and motor deficits. Cranial nerve palsies caused by CMV, especially in the recurrent laryngeal nerve, have been reported in the setting of severe immunosuppression [17]. This symptom may occur with other manifestations of CMV (e.g. polyradiculopathy, encephalitis, or retinitis). Pathologic findings in peripheral nerve biopsies have shown endoneurial necrosis with cellular infiltrates and Schwann cells showing CMV inclusions.
Gastrointestinal system
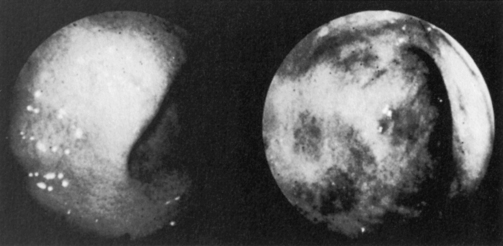
CMV colitis may occur in at least 5–10% of untreated AIDS patients. Diarrhea, weight loss, anorexia, and fever are often present. The differential diagnosis includes infection by other gastrointestinal pathogens, including Cryptosporidium, Giardia, Entamoeba, mycobacteria, Shigella, Campylobacter, and Strongyloides stercoralis, and involvement by lymphoma or Kaposi’s sarcoma. Endoscopy usually reveals diffuse submucosal hemorrhages and mucosal ulcerations, although a grossly normal-appearing mucosa may be encountered in up to 10% of those with histologic evidence of CMV colitis (Fig. 34.2). Biopsy reveals vasculitis, neutrophilic infiltration, and non-specific inflammation, but the diagnosis is confirmed by the presence of characteristic CMV inclusions, antigen, or nucleic acid and the absence of other pathogens.
The efficacy of anti-CMV treatment in patients with enterocolitis is not dramatic [19]. When compared with placebo, a significant antiviral effect was observed, but a clinical benefit was less apparent. Diarrhea and abdominal discomfort were not relieved, but in general, patients seemed to improve with this therapy [19].
Patients with symptomatic esophagitis or enterocolitis who have CMV (and no other pathogens) detected by endoscopy, histology, or culture should benefit from anti-CMV treatment for 3–6 weeks and should be considered for continued maintenance treatment, in part to prevent retinitis [20].
Pulmonary system
Anti-CMV therapy should be considered when a patient has documented CMV pulmonary infection as the only pathogen identified and a progressive, deteriorating clinical course [21–23].
Treatment of CMV infection
Ganciclovir/valganciclovir
Structure and mechanism of action
Ganciclovir is a nucleoside analog that differs from acyclovir by a single carboxyl side chain. This structural change confers approximately 50 times greater activity than acyclovir against CMV. Acyclovir has low activity against CMV because it is not well phosphorylated in CMV-infected cells. This is due to the absence of the gene for thymidine kinase (TK) in CMV. Ganciclovir, however, is active against CMV because it does not require TK for phosphorylation. Instead, another viral-encoded phosphorylating enzyme (UL 97) is present in CMV-infected cells [24]. It is capable of phosphorylating ganciclovir and converting it to the monophosphate. Cellular enzymes then convert the monophosphate to the active compound, ganciclovir triphosphate. Ganciclovir triphosphate acts to inhibit the viral DNA polymerase. Valganciclovir is an oral prodrug of ganciclovir, i.e. it is converted to ganciclovir after absorption.
Pharmacology and dosage
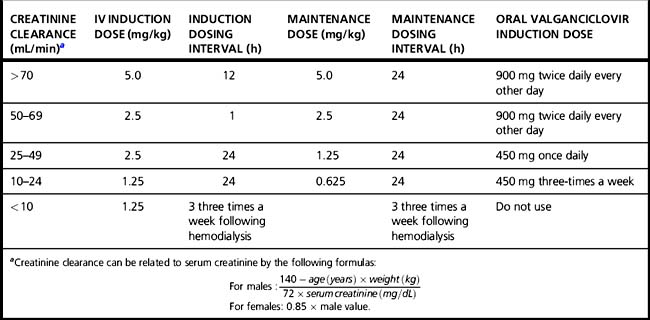
When administered by intravenous infusion over 1 h in the usual dosage of 5 mg/kg, peak ganciclovir blood levels are approximately 8–9 μg/mL, and the serum half-life is 3.5 h. The absolute bioavailability of oral valganciclovir capsules is approximately 60%. When administered orally as 900 mg with food, peak serum levels approach those achieved by intravenous administration of 5 mg/kg. Oral valganciclovir may be used for induction treatment and is the drug of choice for maintenance therapy [25]. Because ganciclovir is excreted unchanged through the kidneys, dosage for intravenous ganciclovir must be reduced in patients with renal impairment. Dosage adjustments should also be considered for oral valganciclovir. The appropriate dose reductions are presented in Table 34.2. Initial response in retinitis (improvement or stabilization in vision or ophthalmoscopic appearance) occurs in approximately 75% of treated patients [6]. By comparison, the disease is relentlessly progressive in 90% of patients if left untreated. Visual-field defects present at the onset of therapy do not reverse, but a decrease in visual acuity caused by edema of the macula may improve with treatment. Retinal detachment may occur in later stages as the necrotic retina scars and thins.
Prior to the availability of HAART, maintenance therapy throughout the life of the patient was critical for CMV retinitis because the virus is only suppressed by ganciclovir and is not eliminated. Even with continued maintenance therapy, CMV retinitis eventually progressed. Why this occurred is not clearly understood, but it is likely related to suboptimal drug delivery to the retina. This hypothesis is supported by the longer times to progression achieved with the ganciclovir intraocular implant, which delivers greater concentrations of ganciclovir to the vitreous [26]. Viral resistance does not appear to be involved in most progressions of CMV retinitis [27].
With successful ART, it is possible for selected patients with CMV retinitis to discontinue maintenance therapy but not until CD4 count has exceeded 100 cells/mm3 for 3–6 months and the retinitis is inactive [28].
Intravitreal injection of ganciclovir has been used in certain special situations, such as in patients in whom neutropenia limited the systemic use of the drug, and in one series [29] appeared effective and relatively safe. Sustained intravitreal release of ganciclovir has been accomplished using a surgical implantable device [14, 30, 31]. This implant, which is designed to deliver ganciclovir into the vitreous over several months, has been shown to be highly efficacious for local control of retinitis. When the implant is used, oral valganciclovir should also be given at least until CD4 function is restored by ART. This is needed to treat or prevent contralateral eye or systemic disease. The implant delivers approximately five times as much ganciclovir compared with intravenous therapy and may be useful in treating low-level GCV-resistant CMV retinitis.
Resistance
Erice and co-workers [32] reported three patients whose clinical course suggested the emergence of resistance and whose CMV isolates exhibited increases in the concentration of ganciclovir required to inhibit the virus in tissue culture over baseline determinations. In a separate report, after 3 months of continuous intravenous ganciclovir therapy, approximately 10% of patients were excreting resistant strains of CMV (arbitrarily defined as strains that are only inhibited by four times or more the median concentration of ganciclovir required to inhibit a group of pretherapy isolates) [33]. In virtually all isolates, there was a mutation in the phosphorylating gene (UL 97) [34]. These strains remain sensitive to foscarnet, which may be used as an alternative therapy. As treatment continues, a polymerase mutation (UL 54) conferring further ganciclovir resistance may occur. Many strains with this mutation are cross-resistant to cidofovir but usually remain sensitive to foscarnet [35, 36].
Toxicity
Effects on hematopoiesis
Leukopenia and anemia may occur in up to 40 and 25%, respectively, of patients receiving intravenous ganciclovir or oral valganciclovir for treatment of CMV disease (Table 34.3). Many patients have low white blood cell counts before therapy, so the contribution of ganciclovir/valganciclovir to leukopenia is not always clear. Neutropenia may develop at any time and is usually reversible. Cytokines, such as granulocyte colony-stimulating factor (G-CSF; filgrastim), are effective in reversing ganciclovir/valganciclovir induced neutropenia. Severe neutropenia (absolute neutrophil count < 500 cells/mm3) requires a ganciclovir/valganciclovir dose interruption until evidence of marrow recovery is observed and neutrophil counts have risen, preferably to > 1,000 cells/mm3. Thrombocytopenia occurs in up to 6% of ganciclovir/valganciclovir-treated patients.
Table 34.3 Selected laboratory abnormalities in patients receiving ganciclovir for treatment of CMV retinitis
| CMV retinitis treatment | IV (5 mg/kg L/d) | Placebo |
|---|---|---|
| Number of patients | 175 | 234 |
| Neutropenia (ANC/L) | ||
| < 500 | 25 | 6 |
| 500–749 | 14 | 7 |
| 750–1000 | 26 | 16 |
| Anemia (hgb, g/dL) | ||
| < 6.5 | 5 | < 1 |
| 6.5–7.9 | 16 | 3 |
| 8.0–9.5 | 26 | 16 |
Data are percentages of patients. ANC; absolute neutrophil count; hgb, hemoglobin.
Toxicities in other organ systems
Gastrointestinal adverse events, most commonly diarrhea, nausea, anorexia, and vomiting, affect a substantial number of patients treated with ganciclovir. When compared to placebo, events are only modestly higher among ganciclovir-treated patients; 48% developed diarrhea (versus 42% of placebo-treated patients), 19% developed anorexia (placebo, 16%), and 14% developed vomiting (placebo, 11%) [37]. In patients receiving valganciclovir, diarrhea occurred in 41%, nausea in 30%, and vomiting in 24%. Neuropathy and paresthesia are the most frequent adverse events involving the nervous system, affecting up to 21 and 10% of patients, respectively, and only neuropathy occurred more often in ganciclovir-versus placebo-treated patients (21% versus 15%, respectively). Neuropathy occurred in 9% of patients receiving valganciclovir. A minority of ganciclovir-treated patients will experience modest elevations in serum creatinine (maximum levels of at least 1.5 mg/dL, or > 25% increases over pretreatment levels).
Foscarnet
Foscarnet is a pyrophosphate that inhibits the DNA polymerase of CMV. Specifically, the drug blocks the pyrophosphate-binding site of the viral DNA polymerase, preventing cleavage of pyrophosphate from deoxyadenosine triphosphate [38]. This action is relatively selective in that CMV DNA polymerase is inhibited at concentrations < 1% of that required to inhibit cellular DNA polymerase. Unlike such nucleosides as acyclovir and ganciclovir, foscarnet does not require phosphorylation intracellularly to be an active inhibitor of viral DNA polymerases. This biochemical fact becomes especially important with regard to viral resistance, because the principal mode of viral resistance to nucleoside analogs is a mutation that eliminates phosphorylation of the drug in virus-infected cells. Foscarnet can be used to treat patients with ganciclovir-resistant CMV; cross-resistance to foscarnet is rare. However, patients treated with foscarnet may develop foscarnet resistance due to mutations in the viral polymerase UL54 [36].
Pharmacology
The recommended dose for initial therapy is 60 mg/kg by intravenous infusion every 8 hours or 90 mg/kg every 12 hours. A dose of 120 mg/kg per day may be superior in efficacy to 90 mg/kg/per day [39], but this dose may also be more toxic.
Palestine and co-workers [5] reported the results of a randomized control trial of foscarnet in the treatment of CMV retinitis in HIV-infected patients. Patients were assigned to receive either no therapy or immediate treatment with intravenous foscarnet. The justification for the design was that the lesions were peripheral and not threatening visual acuity. The mean time to progression of retinitis was 3 weeks in the control group versus 13 weeks in the treatment group, thereby demonstrating the effectiveness of foscarnet. Also, an excellent antiviral effect was achieved in the treatment group (i.e. 9 of 13 patients had positive blood cultures for CMV at entry, and all nine had CMV cleared from their blood by the end of the 3-week induction period). Adverse effects were seizures, hypomagnesemia, hypocalcemia, and elevated serum creatinine levels.
Ganciclovir and foscarnet
The results of a Studies of Ocular Complications of AIDS (SOCA) trial of combination therapy versus monotherapy for relapsed CMV retinitis were published in early 1996 [40]. Combination therapy (5 mg/kg per day ganciclovir and 90 mg/kg per day foscarnet) was significantly superior in delaying progression than either ganciclovir alone (10 mg per day) or foscarnet alone (120 mg/kg per day). This study also found no advantage in switching monotherapy. That is, patients in whom monotherapy failed with ganciclovir who then switched to high-dose foscarnet did not do better than patients who continued ganciclovir at the higher dose. The median times to progression were: foscarnet group, 1.3 months; ganciclovir group, 2.0 months; and combination group, 4.3 months (p < 0.001). Side effects were not statistically significantly different in any group, but the quality of life was poorest in the combination group as a result of the prolonged daily infusion time of 3.1 hours.
Cidofovir
Cidofovir represents a departure from previous nucleoside analogs because it appears to the cell as a nucleotide. It has a phosphonate moiety attached to a cytosine analog and does not require phosphorylation by viral-encoded enzyme. It is therefore active against the majority of ganciclovir-resistant CMV strains that only have resistance mutations in UL97, the phosphorylating gene. When polymerase, UL54, mutations occur in ganciclovir UL54 treated patients, cross-resistance to cidofovir is frequent. These UL54-resistance mutations also occur in patients treated with cidofovir de novo [36]. The drug also has an extremely long half-life, permitting intravenous administration as infrequently as every 2 weeks during maintenance treatment [41].
Immune recovery uveitis
Immune recovery uveitis is an immunologic reaction to CMV characterized by inflammation in the anterior chamber or vitreous. It occurs after the initiation of ART and typically occurs in patients who experience a substantial rise in CD4 count during the 4–12 weeks after initiation of therapy. Treatment consists of periocular corticosteroids or a short course of systemic steroids [42].
Herpes Simplex Virus
Herpes simplex virus types 1 and 2 (HSV-1, HSV-2) cause disease in both normal and immunocompromised hosts and are responsible for substantial morbidity in patients with AIDS. Most adult patients with AIDS have been infected with one or both HSV types before the development of AIDS, and these patients are not susceptible to primary HSV infection following new exposure. During initial HSV infection, viral latency develops in the nerve root ganglia corresponding to the site of mucocutaneous inoculation. Latent virus can then reactivate at any time throughout the life of the host, and all infected persons are at risk for virus shedding and recurrent symptomatic disease. Recurrent HSV mucocutaneous eruptions are common in patients with HIV infection and can be severe, with extensive tissue destruction and prolonged viral shedding [43–45].
Recent studies confirm the high prevalence of both HSV-1 and HSV-2 in the general population [46–48]. Type-specific serologic studies conclude that up to 70% of the population are infected with HSV-1, and up to 22% are infected with HSV-2. HSV-2 infection rates are higher in women than in men and higher in African-Americans and Mexican-Americans than in Caucasians [46–48]. The presence of underlying HSV infection may increase the risk of acquiring HIV infection following exposure to HIV. This increased risk for HIV may occur as a result of the presence of susceptible CD4 T cells present in HSV ulcerations [49, 50]. The prevalence of HSV infection in homosexual AIDS patients exceeds that of the general population and likely reflects the common risk factor for transmission of both HSV and HIV (sexual contact). Serologic studies have revealed that up to 77% of HIV-infected patients have been previously infected with HSV. AIDS subgroups who did not acquire HIV infection through sexual contact, such as hemophiliacs and transfusion recipients, have rates of HSV infection that are lower than the incidence in patients with AIDS as a whole, and are likely comparable with those in the general population. The presence of latent HSV infection in this high percentage of patients with HIV infection explains the frequency of clinical disease in this population. Clinical observations suggest that the frequency and severity of HSV recurrences may increase with advancing immunosuppressions [48, 51–54].
Clinical presentation
Because most HIV-infected patients have been infected with HSV before acquiring HIV, recurrent HSV is much more common than primary HSV infection in this population. HSV infection in AIDS patients may appear similar to the typical HSV lesions observed in the normal host or, alternatively, lesions may appear quite atypical and unusual because of the immunosuppressed state associated with HIV infection. The severity of clinical illness depends on several factors, including the anatomic site of initial infection, the degree of immunosuppression, and whether the clinical episode represents initial primary infection (no previous exposure to either HSV type), initial non-primary infection (previous exposure to the heterologous HSV type), or recurrent infection [43–45].
Orolabial infection
The incubation period of primary HSV infection ranges between 2 and 12 days. In the normal host, primary orolabial infection may be asymptomatic or result in clinically apparent gingivostomatitis [45–48, 55]. Immunocompromised patients are at greater risk than normal hosts of developing a severe clinical illness during primary HSV-1 infection, with a painful vesicular eruption occurring along the lips, tongue, pharynx, or buccal mucosa. The vesicles rapidly coalesce and rupture to form large ulcers covered by a whitish yellow necrotic film [55, 56]. Fever, pharyngitis, and cervical lymphadenopathy are often present in adults, whereas infants may display poor feeding and persistent drooling.
Following initial or primary infection, all infected patients remain at risk for virus reactivation and recurrent disease. Recurrent HSV gingivostomatitis (“fever blisters”) may occur spontaneously or as a result of external stimuli, such as a febrile illness, excessive wind or ultraviolet light exposure to the lips, surgical manipulation of the trigeminal nerve, or stress. Prodromal symptoms, consisting of tingling or numbness at the site of the impending recurrence, may be present from 12 to 24 hours before the onset of an HSV recurrence. Instituting antiviral therapy during the prodrome may shorten the duration of illness or may abort the development of visible cutaneous lesions (see Treatment of HSV Infection, below). Recurrences may increase in frequency and severity as immunosuppression worsens, although many AIDS patients will have only infrequent, mild, self-limiting recurrences throughout the course of their disease [51, 55].
In the normal host, orolabial herpes lesions usually heal in 7–10 days. By comparison, AIDS patients often have a prolonged illness with markedly delayed healing of mucocutaneous lesions. If left untreated, chronic ulcerative lesions with persistent viral shedding may last for several weeks [56].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree