Chapter 39 Life-threatening emergencies
Learning outcomes for this chapter are:
1. To discuss those factors that indicate a potential for emergency situations for mother and baby in pregnancy, birth and the puerperium
2. To describe the aetiology and management of a range of emergency situations in pregnancy, labour, birth and the puerperium
3. To apply best available evidence in the identification, assessment and collaborative management of umbilical cord presentation and prolapsed cord, shoulder dystocia, postpartum haemorrhage, eclampsia, amniotic fluid embolism, uterine inversion, maternal trauma, stabilisation, cardiopulmonary resuscitation and transport, and neonatal resuscitation.
artificial rupture of the membranes
disseminated intravascular coagulation
umbilical cord (funic) presentation
For most women, childbirth is a healthy event. However, some women may develop complications requiring complex levels of medical and technological intervention, and it is these women who are the focus of this chapter. Healthcare practitioners must ensure that their practice is current, skilled and based on the best available evidence. This chapter is concerned with the most frequent life-threatening complications, which often transpire with little or no warning. It is not a definitive guide to the management of midwifery/obstetric emergencies. A thorough understanding of the biosciences and pathophysiology underpinning emergency conditions in midwifery is crucial to best practice. Therefore, readers will need to supplement the information they gain from this chapter with specialised texts such as Blackburn (2007) and/or the second edition of Stables and Rankin (2005). In addition, Chapter 23 provides an excellent overview of the physiological changes that occur during labour.
The emergency management of eclampsia is included in this chapter because it is a life-threatening emergency. Readers are encouraged to review Chapters 37 and 38, which include descriptions of the pathophysiology and management of hypertension in pregnancy, before proceeding to sections on eclampsia.
INTRODUCTION
Researchers have developed a number of screening tools to help clinicians make accurate predictions about which women are most at risk of complications in pregnancy and birth, with limited success. Birth is never 100% predictable, and hence the rationale underpinning the policy that there should be a skilled attendant at every birth. Worldwide, between 9% and 15% of all birthing women will experience potentially life-threatening complications, and at least 1%–2% will need a major intervention to survive (Maine et al 1992). Midwives have a statutory responsibility to adhere to the principles outlined by their professional and regulatory authorities. Australia’s Competency Standards for Registered Midwives (ANMC 2006), the Midwifery Council of New Zealand’s Competencies for Registration as a Midwife (MCNZ 2004) and the Standards for Practice (NZCOM 2008) are all grounded in a primary healthcare model of practice that is influenced by particular sociocultural, spiritual and politico-economic environments. For example, the midwife ‘respects and supports the needs of women and their families/whānau to be self-determining in promoting their own health and well-being’ (MCNZ 2004, p 17). According to both Australia’s and New Zealand’s competency standards, the midwife accepts accountability and responsibility for her actions while recognising her own knowledge base and scope of practice. She is able to identify complications, with appropriate and timely consultation and referral as needed. The midwife delegates when necessary, always providing the appropriate supervision, and collaborates with other healthcare providers when care is outside her scope of practice.
SCENARIO-BASED CLINICAL SKILL TRAINING—‘FIRE DRILLS’
• Preconception care—better counselling and support to be provided for women, especially those with preexisting serious medical or mental health problems such as epilepsy, diabetes and obesity (body mass index BMI > 30).
• Access to care—antenatal services must be accessible and welcoming to women. Women should have had their first visit to the antenatal clinic after the first 12 completed weeks of pregnancy.
• Migrant women—the medical history and clinical assessment of overall health for these women must be recorded. Healthcare professionals should be particularly sensitive towards women from countries where genital mutilation is practised and provide appropriate care for them.
• Treatment for systolic hypertension—pregnant women with a systolic blood pressure ≥ 160 mmHg must be provided with anti-hypertensive treatment.
• Caesarean section—women who have had a previous caesarean section or are going to have one should be advised about the risks in future pregnancies.
• Clinical skills—trusts and practices should instil a learning culture from critical events and serious untoward incidences (SUIs). Additional staff training for care to newborns should be provided.
• Early warning scoring system—trusts should adopt an early warning system to help in the timely recognition, treatment and referral of treatment of women who have or are developing critical conditions.
• Guidelines for development—guidelines for the care of women who are obese, have sepsis during pregnancy, and pain and bleeding during early pregnancy should be produced.
There is documented evidence (Draycott et al 2008) that simulated training in obstetric emergencies reduces the likelihood of avoidable errors during emergency situations. It is imperative for all midwives, especially those in remote rural locations, to have ‘crash’ protocols in place and to practise scenario-based clinical ‘fire drill’ skills training to facilitate effective management of emergencies. The aims of these ‘fire drills’ are to test local systems and protocols for responding to emergencies, and to facilitate interdisciplinary teamwork and the development of individual skills and knowledge. ‘Fire drills’ should include the procedures to be taken in the event of activation, calls for help, first-level management, staff responses, and home/birth centre/labour ward/operating logistics. The ‘fire drill’ should be initiated and directed by the midwife directly responsible for the conduct of the emergency and should be supported by the multidisciplinary team members. This includes the woman’s right to be involved in decisions about her care. As soon as possible during and after any emergency, the woman and her family should be provided with an explanation of the circumstances and the need for urgent action. What we do and how we do it must be informed by evidence, and unfortunately obstetrics and midwifery are still ‘inexact sciences’.
DECISION TO (OPERATIVE) DELIVERY
In an emergency, the time from the decision to deliver by caesarean section and the actual delivery of a baby is known as the ‘decision to delivery’ (D–D) interval. There are no randomised clinical trials demonstrating that the faster a caesarean section is performed, the better the maternal and fetal outcome. The accepted interval of 30 minutes was first recommended in a Canadian consensus statement in 1986 (Hannah et al 1986), even though there was and still is little evidence to support it. Several other studies examining the association between D–D interval and maternal and baby outcomes have found little difference between babies born with a D–D interval of 15 minutes and those with a D–D interval between 16 and 75 minutes (Thomas et al 2004). Indeed, research has found that fewer than 40% of caesarean sections for fetal compromise were achieved within 30 minutes of the decision (MacKenzie & Cooke 2001). Further, there was ‘no evidence to indicate that overall an interval up to 120 minutes was detrimental to the neonate unless the delivery was a “crash” caesarean section’ (MacKenzie & Cooke 2001, p 498). ‘Crash’ caesarean sections increase the risk of gastric aspiration during general anaesthesia. Morbidly obese women are at especially high risk of this complication (Saravanakumar et al 2006).
Clinicians are now aware that ‘anaesthetic rush often leads to obstetric crash’. Women have died from anaesthetic complications during attempts to perform a crash caesarean section (Saravanakumar et al 2006). The authors state that anaesthetic complications that have caused death include inability to maintain a patent airway, and vomiting resulting in aspiration and hypoxaemia. For a woman who is transferred from home or a birth centre, there is some risk that her stomach is not empty and general anaesthesia is especially risky in this setting. (In some cases, the risk of complications associated with general anaesthesia can be reduced with spinal anaesthesia.) In most cases there is ample time for an anaesthetist to prepare the woman for the caesarean section. Therefore, significantly reducing D–D times may actually cause more harm than good.
WOMEN’S FEELINGS AND FEARS
Women can be deeply scarred by a traumatic birthing. Australia’s Birthrites website (www.birthrites.org/) is devoted to women seeking a vaginal birth after a previous caesarean section. The New Zealand TABS website (www.tabs.org.nz) provides support for women after a traumatic birthing experience as well as education for healthcare professionals.
Mapp and Hudson have published their findings of research about work in this area in two articles in the British Journal of Midwifery. The first (Mapp & Hudson 2005a) reports the findings of a qualitative study using a phenomenological approach which explores women’s experiences of obstetric emergencies. Selection criteria were specifically aimed at women who had experienced one or more of the following types of emergencies during childbirth: cord prolapse, placental abruption, shoulder dystocia, uterine scar rupture, severe preeclampsia and major postpartum haemorrhage. The 10 women interviewed by the researchers described their ‘lived experiences’ of the emergencies. Significant themes included issues around communication, both verbal and non-verbal, and the need to make sense of what had happened to them. The findings provide a valuable insight into obstetric emergencies, and highlight areas for improvement as well as the positive aspects to care that women received during these traumatic and often life-threatening events. The authors provide some examples of strategies aimed at ‘minimising’ the emotional trauma associated with traumatic events: one member of the team assumes the role of being ‘with the woman’ to provide emotional support, i.e. touch (hand holding), a smile of reassurance, giving simple and brief explanations without medical jargon and informing her that a detailed explanation will be given when the crisis is over (Mapp & Hudson 2005a, p 34). The second article by the same authors deals with ‘debriefing’.
CORD PRESENTATION AND PROLAPSE
Umbilical cord prolapse constitutes an obstetric emergency. It occurs in about 1 in 1000 births with up to a 50% perinatal mortality rate (Curran 2003). In many cases it is iatrogenic and thus a preventable condition. The risks increase whenever the presenting part does not fit snugly into the pelvis. Although a prolapsed cord poses no immediate physical threat for the woman, interventions to save the fetus, such as an emergency operative birth, will threaten her safety and wellbeing. Moreover, an unexpected emergency situation combined with fear for the wellbeing of her child causes immense stress for the woman and her family.
Definitions
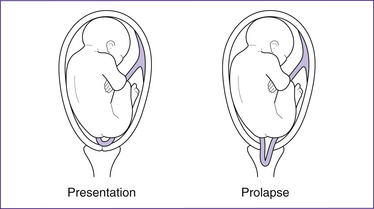
Umbilical cord presentation
An umbilical cord presentation (Fig 39.1) is a condition in which the umbilical cord is interposed between the leading part of the fetus and the internal os of the uterine cervix but the amniotic membranes remain intact. Once the membranes break, the risk that the cord will prolapse through the cervix into the vagina increases significantly. In an occult cord presentation, a loop of cord lies alongside the presenting part and may not be felt on vaginal examination. A non-reassuring fetal heart rate (FHR) trace with deep, early decelerations may be an early sign of this complication. As labour advances, intermittent umbilical cord compression causes variable FHR decelerations. Once the membranes break, the umbilical cord usually prolapses through the cervix and is compressed with every contraction, causing severe variable FHR decelerations and/or profound fetal bradycardia.
Umbilical cord prolapse
Cord prolapse has been defined as descent of the umbilical cord through the cervix alongside (occult) or past the presenting part (overt) in the presence of ruptured membranes. An umbilical cord that, in the presence of ruptured membranes, lies in front of or beside the presenting part is said to be prolapsed (Fig 39.1). It occurs when the cord falls or is washed down through the cervix into the birth canal; it may be visible at the introitus. An occult prolapse occurs when the membranes break and the cord becomes trapped between the presenting part and the walls of the maternal pelvis. Thus, it is most likely to occur if the pelvic inlet is not completely filled by the presenting part.
Table 39.1 Incidence of cord prolapse
| Overall risk in vertex presentations | 0.2–0.5% |
| Overall risk in breech presentations | 3.5% |
| Complete breech | 2–5% |
| Footling breech | 15% |
| Transverse lie | 20% |
| Multiple pregnancies | 4% |
| Preterm labours and birth | 5–10% |
(Source: Gabbe et al 2002)
apparent and the latter is usually diagnosed by sonography or during a ‘routine’ vaginal examination of a clinically asymptomatic woman. Should the umbilical cord prolapse through the cervix or even lie between the presenting part and the pelvis, during uterine contractions it will be exposed to intermittent compression, which compromises the fetal circulation. Depending on its duration and the degree of compression, fetal hypoxia, brain damage and even fetal death may occur. Dramatic changes in the fetal heart rate are often the first signs of a cord prolapse.
Incidence
The overall incidence of cord prolapse ranges from 0.1% to 0.6% (Lin 2006). In the case of breech presentation, the incidence is slightly more than 1% (Dilbaz et al 2006). It is very difficult to predict impending cord prolapse. First, ‘routine’ ultrasound examinations are not sufficiently sensitive or specific for identification of cord presentation and should not be performed to predict increased probability of cord prolapse (Ezra et al 2003). Second, about half of electronically monitored labours show typical fetal heart rate (FHR) changes suggestive of umbilical cord presentation. The phenomenon is usually transitory and relieved by changing the maternal position. Third, approximately 50% of prolapsed cords occur in the second stage of labour. Significantly, 47% are iatrogenic resulting from some obstetric intervention such as amniotomy or following external cephalic version (ECV) (Usta et al 1999).
Risk factors
According to Dilbaz (2006), the risks of umbilical cord presentation and prolapse increase in frequency with malpresentation and with poorly fitting presenting parts, such as in a transverse lie, shoulder or breech presentation. A flexed or footling breech poses the most risk for cord prolapse due to the increased space through which the umbilical cord can fall into the vagina. Prematurity is also a risk factor for cord prolapse because preterm babies are more likely to present as a malpresentation, and their small size in relation to the maternal pelvis means there is more room for loops of cord to escape through the cervix. Additionally, there is also more liquor volume in relation to fetal size than for a term infant.
Any structural abnormalities of the placenta, uterus or pelvis may also stop the presenting part from fitting snugly into the pelvis and thus boost the risk of prolapse (e.g. succenturiate placental lobe or contracted pelvis). A low-lying placenta (placenta praevia) can prevent the presenting part from entering the brim of the pelvis and it too adds to the risk. Repeated pregnancies interfere with the integrity of the abdominal musculature, which means that some multiparous women have lax abdominal muscles, which increases the likelihood of an ill-fitting presenting part at term. Finally, rarely the use of external cephalic version (ECV) to prevent breech presentation at birth is rarely associated with cord prolapse, so the procedure should ideally be conducted in an environment where fetal wellbeing can be assessed and resources are available to proceed to an emergency caesarean section (see also Chs 37 and 38).
Whenever the presenting part is not fully engaged, an amniotomy (artificial breaking of the membranes)—especially in the presence of polyhydramnios—poses a real risk of an iatrogenic cord prolapse. Of course, spontaneous rupture of the membranes can also trigger cord prolapse and so it is important for the midwife to auscultate the fetal heart prior to and immediately after amniotomy and as soon as is practical following a spontaneous rupture of membranes. A review of the use of ‘routine’ amniotomy can be found in Chapter 37.
Diagnosis during a vaginal examination
Box 39.1 Clinical diagnosis of cord presentation and prolapse
• Cord presentation—loops of cord are palpated through the membranes. The presence of umbilical cord lying in front of the presenting part can also be visualised using colour Doppler studies.
• Cord prolapse—diagnosis is made by visual inspection or palpation of a pulsing umbilical cord during a vaginal examination. The umbilical cord is felt below or beside the presenting part.
• Occult prolapsed cord—is rarely felt on vaginal examination and the only indication may be FHR changes.
• Overt prolapse—the umbilical cord can be seen protruding from the introitus or loops of cord palpated within the vaginal canal.
fetal bradycardia. Because of the association between cord prolapse and fetal bradycardia and/or or severe variable or prolonged FHR decelerations, the presence of such decelerations should prompt vaginal examination.
The midwife may also diagnose vasa praevia during a vaginal examination. This is a very rare condition where the umbilical cord vessels covered only by chorion and amnion lie across the cervical os and ahead of the fetus. In this situation an amniotomy is contraindicated as the cord has very little protection and the vessels often tear as the membranes break, causing asphyxia, exsanguination and fetal death.
Management of cord presentation in labour
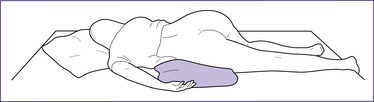
The immediate aims are to relieve pressure on the cord to ensure an adequate oxygen supply to the fetus and to prevent cord prolapse, by repositioning the woman in either the exaggerated Sims’ position (see Fig 39.2) or in the head-down bottom-up position. The goal is to birth the baby as soon as possible; usually this is by caesarean section and in a manner that provides safe anaesthesia for the mother. If the woman has a working epidural in place, there may be time for this to be topped up, by the anaesthetist, either before transportation or en route to theatre, thus avoiding general anaesthesia. If the woman has had a spinal block, it is important not to position the woman in a way that leads to a ‘high block’ and a possible respiratory arrest. Sometimes a general anaesthetic is the method of choice, perhaps because a skilled anaesthetist is not available.
Administering oxygen at 6–8 L/min may help to prevent fetal hypoxia.
Management of cord prolapse in labour
• cord compression preventing venous return to the fetus
• umbilical arterial vasospasm secondary to exposure to vaginal fluids and/or air.
Even short delays in treatment are associated with significant perinatal morbidity and mortality. In brief, if the fetus is viable and the cervix is not completely dilated, prompt delivery via caesarean section offers the best chance for a favourable fetal outcome. Effective elevation of the presenting part out of the pelvis, along with tocolysis, removes the urgency of an immediate caesarean section. A vaginal birth may still be possible if the birth is imminent—that is, the cervix is completely dilated, the presenting part is well down and there are no contraindications that will slow the birth. If the fetus is very preterm or is dead, nature should take its course and labour should proceed to a vaginal birth. Fetal death is confirmed with an ultrasound scan.
First-level strategies (until help arrives)
The following strategies are designed to improve oxygen delivery to the fetus. If the fetus is viable and birth is not imminent, the woman is asked to assume an exaggerated Sims’ position (her hips can be elevated on pillows; see Fig 39.2) and the midwife inserts (or keeps) two fingers into her vagina to push the presenting part up and away from the cord. A variation is to remove the hand from the vagina once the presenting part has been pushed above the pelvic brim and continuous suprapubic pressure is applied upwards. Excessive displacement may encourage more of the cord to prolapse. This manoeuvre is maintained until help arrives or the baby is born. If the cord is visible at the introitus it should be replaced gently in the vagina, to minimise chilling and vasospasm. An alternative recommendation is to gently wrap the cord in warm saline-soaked swabs to prevent reactive vasoconstriction and the onset of fetal hypoxia-acidosis.
Summary—first-level management
• Change the mother’s position.
• Note time of diagnosis of cord presentation/prolapse and maintain a contemporaneous record of events until birth occurs.
• Minimise compression of the cord between the fetal head and the pelvis.
• Administer 100% oxygen via a Hudson mask at 6–8 L/min. Note that there is no recent, good-quality evidence that supports the efficacy of giving supplemental oxygen, but neither is there any evidence to suggest that it causes harm.
• Give a brief explanation to the woman and her family.
• Prepare the woman for theatre and an operative delivery unless birth is imminent or the fetus is not viable or is dead.
Second-level strategies
Critical thinking exercise
• how to identify risk factors for umbilical cord prolapse
• how to recognise a cord presentation in a timely way
• why an ARM should not be performed without a clinical indication
• what you can do if a clinician tells you he or she wishes to perform an amniotomy when the fetal head is still 4/5 above the brim of the pelvis.
and fetal circulation. This is why it is critical that the midwife’s first action must be to minimise compression of the umbilical cord. There is now good evidence to show that in most cases a calm unhurried approach, alleviating cord compression together with the administration of 100% oxygen to the mother followed by an operative delivery (usually by caesarean section with general anaesthesia) minimises complications for mother and baby.
• Depending upon local circumstances, some procedures (e.g. intravenous cannulation, blood tests for ABO and Rh testing, and urinary catheterisation) can be delayed until the woman is in the operating theatre.
• The usual prophylaxis to prevent the adverse effects of the aspiration of gastric contents should still be given to the woman, e.g. sodium citrate plus ranitidine.
If the D–D interval is likely to be prolonged, particularly if it involves ambulance transfer, umbilical cord compression may also be relieved by first pushing the fetal head out of the pelvis and then inserting a Foley catheter into the maternal bladder which is filled with normal saline. This can be achieved quickly by inserting the end of a blood-giving set into the catheter. The catheter should be clamped once 500–750 mL of normal saline has been instilled. Transfer via ambulance with a very full bladder can be very uncomfortable. It is essential to empty the bladder just before delivering the baby, whether by vaginal birth or caesarean section (Griese & Prickett 1993; Runnebaum & Katz 1999). Tocolysis is a useful adjunct therapy using obstetric salbutamol or terbutaline (see below).
Out-of-hospital births
Even if a vaginal examination by the lead midwife reveals that a spontaneous vaginal birth is imminent, preparations for transfer should still be made because it is likely that the baby will require active resuscitation. If birth is not imminent, the presenting part should be elevated during transfer by either manual or the bladder-filling methods (see above). It is a good idea for community-based midwives to carry a Foley catheter for this purpose and equipment for fluid infusion.
In a hospital setting, if immediate vaginal birth is not possible it is also important to inhibit uterine contractions. Synthetic oxytocin (Syntocinon) infusions are turned off if being used and, if an IV line is already in place, the rate of
IV infusion of Hartmann’s solution is increased. Tocolysis with a drug such as terbutaline or obstetric salbutamol has been used successfully to inhibit uterine contractions and thus further reduce compression on the umbilical cord (American Academy of Family Physicians 2000).
SHOULDER DYSTOCIA
Shoulder dystocia, ‘bed’ dystocia and ‘mild’ dystocia
Box 39.2 Clinical review
The ‘cardinal movements of labour’ for a vertex presentation
• The bisacromial diameter of a term fetus is greater than the biparietal diameter.
• The pelvic inlet is wider in the oblique diameter than the anteroposterior diameter.
• Unless it has occurred already, during labour uterine contractions lead to flexion, descent of the fetal head and engagement.
• Usually the fetal head enters the pelvic inlet in the occipitolateral position, with the shoulders lying in the anteroposterior diameter.
• Internal rotation of the head occurs as the head reaches the level of the ischial spines and the shoulders rotate to the oblique position.
• The fetal head crowns and extends as it passes through the pelvic outlet.
• The shoulders now pass through the pelvic inlet in the oblique diameter of the pelvis. The posterior shoulder enters first, coming to rest in the sacral hollow or over the sacrosciatic notch, while the anterior shoulder follows it to lie over the obturator foramen.
• As further descent occurs, the anterior shoulder emerges from under the pubic ramus, and the shoulder girdle rotates to allow delivery in the anteroposterior position.
Incidence and risk factors
Most cases occur in fetuses of normal birthweight and are unanticipated, limiting the clinical usefulness of risk-factor identification. Approximately half occur without warning and in the absence of any known risk factors. Box 39.3 lists thee antenatal and intrapartum risk factors for shoulder dystocia.
About half of all shoulder dystocias occur in macrosomic babies (Baxley & Gobbo 2004; Haram et al 2002). As birthweight increases, so does the occurrence of shoulder dystocia—occurring in 0.6%–1.4% of births where the infant weighs between 2500 g and 4000 g; in infants with a birthweight of 4000–4500 g, the incidence increases to 5%–9% (Baxley & Gobbo 2004).
Anecdotal, but not research-based, evidence suggests that women are increasingly choosing to have caesarean sections because they have been told by their lead caregiver that their baby is so big that they are at significant risk of shoulder dystocia. Women (and clinicians) need to know that most babies with a birthweight of ≥4500 g do not develop shoulder dystocia (Naef & Martin 1995) and, just as importantly, in 48% of cases shoulder dystocia occurs in infants with a birthweight of less than 4000 g (Baskett & Allen 1995).
Summary of risk factors for shoulder dystocia
• Large fetus—even in the presence of a well-sized maternal pelvis, shoulder dystocia can occur during delivery if the fetus is macrosomic. A macrosomic fetus is defined as one with a weight greater than 4000 g.
• Small maternal pelvis—shoulder dystocia can occur if the dimensions of a woman’s pelvis are smaller than those accepted as normal. Under these circumstances, delivering a normal fetus becomes difficult.
• Maternal obesity (BMI >35) is a known cause of fetal macrosomia or a large fetus (described earlier).
• Diabetes mellitus—maternal diabetes mellitus is a leading cause of fetal macrosomia.
• Prolongation of late first stage of labour.
• Prolonged second stage of labour.
Even in the presence of one or more of the above risk factors, more women have uneventful birthing experiences than those whose labours are complicated by shoulder dystocia. Moreover, shoulder dystocia can occur in the absence of these factors. Additionally, brachial plexus injuries can occur without the occurrence of shoulder dystocia.
Maternal diabetes, macrosomia and shoulder dystocia
Currently, prevention of shoulder dystocia in diabetic women is based on quality antenatal care. Control of blood sugar levels reduces the incidence of fetal macrosomia. A review in the Cochrane Database (Boulvain et al 2000) reveals no firm evidence to support elective delivery, by induction of labour or by elective caesarean section, over expectant management at term for non-insulin-requiring diabetics. In other words, labour induction for suspected fetal macrosomia does not lower the rates of shoulder dystocia or caesarean delivery. In the United States, analytical decision models have estimated that 2345 caesarean deliveries, at a cost of nearly US$5 million annually, would be needed to prevent one permanent brachial plexus injury in a patient without diabetes who had a fetus suspected of weighing more than 4000 g.
Nonetheless, according to Boulvain et al (2000) elective caesarean section should be considered as a means of reducing the potential morbidity for pregnancies complicated by insulin-dependent diabetes and by suspected fetal macrosomia. The authors also acknowledge that while timely induction of labour in women with
gestational diabetes who require insulin potentially reduces the risks associated with macrosomia, the actual risk of maternal or neonatal injury is not modified.
Maternal obesity and shoulder dystocia
In a study of pregnancy complications and adverse perinatal outcomes associated with obesity, Cedergren (2004) found that shoulder dystocia occurred three times more often in overweight women than in those of ‘normal’ weight. Morbidly obese women are more at risk for having a caesarean section; it is speculated that the connection may be due to increased deposits of soft tissue in the maternal pelvis which impede vaginal delivery. Obese women are more at risk for complications from operative deliveries such as venous thromboembolic events, wound infections or complications from the anaesthetic. Researchers have found that maternal obesity is not a significant independent risk factor for shoulder dystocia once risk factors are controlled for fetal macrosomia, diabetes, previous macrosomic infants and midpelvic instrumental vaginal delivery. Therefore ‘elective’ caesarean section is not indicated as a prophylactic measure (see also Ch 35).