Anatomy of the breast
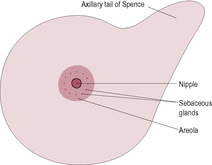
Fig. 16.1
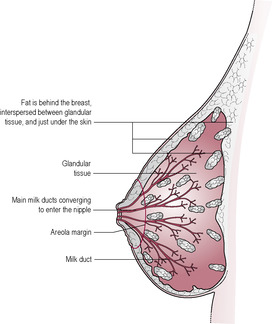
Fig. 16.2
Breast growth and development
Physiology of lactation
Source
Anterior Pituitary Gland
Posterior Pituitary Gland (but Synthesized in Hypothalamus)
Primary control
Lifting of dopamine inhibition
Neural pathway
Modulating factors
Positively stimulated by oestrogen, TSH, VIP
Neurotransmitters
Peak response
30 min
30 s
Stimulus
Suckling
Suckling, sound, sight and thought of baby
Target cell
Alveolar cell
Myoepithelial cell
Effect
Milk synthesis
Milk ejection
Prolactin
Biosynthesis of milk
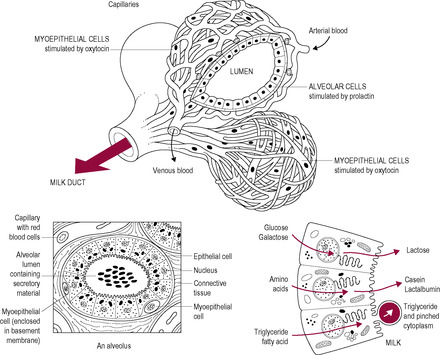
Fig. 16.3
Oxytocin
Suckling and milk transfer
Involution
Problems associated with lactation
Milk insufficiency
Drugs
Psychological stress and breast diseases
Viruses
Inhibition of fertility
Maternal behaviour
Nutrition of the lactating mother
Energy requirements for milk production
Minerals
Water and fluids
Infant nutrition and the composition of human milk
Colostrum
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Lactation and infant nutrition
Almost immediately following his birth, Zara placed Zak in her arms and he instinctively latched onto the breast and started feeding before the cord was cut. Zak fed on the breast for over 45 min before detaching himself.
• What are the advantages of offering the baby the breast as soon as possible after delivery for both mother and baby?
• What factors could have a negative influence on the early establishment of breastfeeding and how can the midwife optimize breastfeeding?
The tissue of the breast extends from about the second to the sixth rib (depending on posture). The extension of the tail of the tissue into the axilla (Fig. 16.1) can result in discomfort in the early puerperium when it may become swollen. The mammary gland is made up of a branching network of ducts ending in lobular–alveolar clusters which are the sites of milk secretion. The breast also has a variable amount of adipose tissue and connective tissue. The breast is divided into sections or lobes by fibrous septae, which run from behind the nipple towards the pectoralis muscle. These septae are important in localizing infections, which are often visually evident as a wedge of red inflamed skin on the surface of the breast. Each of the 8–12 lobes, separated by connective tissue, contains glandular tissue composed of clusters of alveoli and small ducts (Fig. 16.2). The alveolar secretory cells are grouped in grape-like lobules around an extensive branching system of small ducts, which lead to the nipple. Fat is interspersed throughout the lobules. Ultrasound studies of babies feeding at the breast show that there are no discernible lactiferous sinuses in the human breast and that the ducts, even close to the nipple, can branch and be very small and compressible (Ramsay et al., 2005).
(Reproduced with permission from Ramsay et al., 2005.)
The nipple is surrounded by the areola, a pigmented area of varying size, which darkens during pregnancy and has a rich vascular supply and sensory nerve inputs. Surrounding the nipple are Montgomery’s tubercles which are sebaceous glands that hypertrophy and become prominent during pregnancy, providing lubrication and protection. Heavy use of soap can increase the risk of nipple damage, particularly drying and cracking. The sensitivity of the nipple and surrounding area increases markedly immediately after delivery. Suckling results in an influx of afferent nerve impulses to the hypothalamus controlling lactation and maternal behaviour.
Each lobe consists of 20–40 lobules, each containing 10–100 alveoli, the glandular physiological units. Alveolar cells are cuboidal in the resting non-pregnant breast and change remarkably to develop full secretory features during lactation. The alveolar cells secrete milk into the lumen of the small ducts. These secretory cells are surrounded by oxytocin-sensitive myoepithelial (contractile) cells, which are important in milk ejection. The ducts are also lined by contractile cells that open the ducts widely during the milk ejection reflex to assist flow.
Mammary growth and development can be divided into four phases: resting, development (pregnancy), milk secreting (lactation) and involution. At birth, the structure is simply the nipple and a few rudimentary ducts, with few or no alveoli, reflecting their evolutionary origin of modified apocrine sweat glands. Until puberty, the only degree of development may be a little branching of the ducts. There is a decreased incidence of breast cancer in populations with a high consumption of phytoestrogens (oestrogen-like compounds derived from plants). It is suggested that the phytoestrogens stimulate development of the mammary cells in childhood and puberty before pregnancy; these well-differentiated cells may be more resistant to tumour formation (Adlercreutz, 1995).
The human is unusual, even compared with other primates, having extensive breast development at puberty, rather than at pregnancy, resulting in an erotic significance. During puberty, the proliferation of the milk ducts, which elongate, sprout and branch, is primarily dependent on secretion of oestrogen with further contributions from growth hormone (GH) and adrenal hormones. The modest alveolar development at this stage is stimulated by progesterone, providing the tissue has been primed by oestrogen. Prolactin may also play a role although the interaction between the adrenal and pituitary glands and the ovaries is not fully understood. The hormonal fluctuations of the menstrual cycle give repeated exposure of the tissue to oestrogen and progesterone, which allow additional but limited growth. Many non-pregnant women experience cyclical changes, especially premenstrually, in breast volume, which is associated with water retention. Occasionally, some secretory activity may occur within the alveoli and a mammary secretion may be expressed premenstrually.
Once an adult woman has developed breasts, minimal stimulation is required to begin milk secretion (Box 16.1). Growth in breast size is most marked in early pregnancy (Hytten, 1995). The hormones required for breast development during pregnancy are less than those required for other species. In humans, neither human placental lactogen (hPL) nor GH is essential.
Box 16.1
Relactation, or induced lactation, is the process whereby lactation is initiated at a time not associated with delivery. For instance, an adoptive mother who has not borne a child may wish to breastfeed her adopted baby or a mother may want to resume feeding her own child. Relactation is easier if the woman has previously lactated or been pregnant and if the infant is young. Hormonal support such as oxytocin nasal sprays may be used. The woman is advised to eat well and rest, and to stimulate the nipple and breast often, either by hand or with a breast pump. Supplementary formula milk is given to the baby by spoon or dropper; bottle teats and dummies are avoided. Use of a ‘Lact-Aid’ supplementer is often found helpful. This device allows the baby to feed on formula milk from a tube attached to the mother’s nipple. As the baby feeds, it stimulates the nipple and increases endogenous prolactin secretion. The formula milk is in a bag maintained at body temperature because it is in contact with the mother’s body. As breast milk production increases, the amount of formula milk can be reduced.
In early pregnancy, breast size and areolar pigmentation increase. The tubercles of Montgomery enlarge and the nipples become more erect. Blood flow to the breast doubles so blood vessels become more prominent and the skin may appear to have a translucent marbled appearance. There is a sharp increase in ductal and glandular elements so the breasts tend to feel slightly lumpy in early pregnancy. This initial hyperplasia is followed by alveolar cell hypertrophy and initiation of secretory activity in later pregnancy.
Oestrogen plays the dominant role in development of the ducts and progesterone in the development of glandular tissue, although insulin and other growth factors, such as epidermal growth factor (EGF) and transforming growth factor (TGFα), have a role in regulation. Changes in pregnancy depend on the lactogenic hormones, prolactin and hPL, with placental oestrogen and progesterone playing important modulatory roles. Under these hormonal influences, prominent lobules, resembling bunches of grapes, form in the breast so the alveolar lumen becomes dilated by mid-pregnancy and the secretory cells fully differentiated (Box 16.2). The areola becomes pigmented and a secondary patchily pigmented areola may develop. The nipple enlarges and becomes more mobile and protractile, as the connective tissue anchorages soften and become more stretchable with the oestrogen-driven increase in hydration (see Chapter 11). By the 4th month of pregnancy, the epithelial cells accumulate substantial amounts of secretory material and the mammary glands are fully developed. Prolactin levels progressively increase throughout the pregnancy and are maximal at term. Although it is possible to expel a breast secretion in pregnancy, this is not true colostrum. Full colostrum and milk production is inhibited by high progesterone levels so copious milk production is not established until after parturition. Placental hPL may also contribute to blocking of prolactin responses in pregnancy. Even if very small amounts of the placenta or fetal membranes are retained after delivery, lactation is inhibited (Neifert et al., 1981) (see Chapters 13 and 14).
Box 16.2
• Increased vascularization – may cause tingling
• Dilatation of superficial veins – fair skin appears ‘marbled’
• Hypertrophy – full development of lobules
• Dilatation of alveoli and ducts – may feel nodular
• Thickening of nipple skin
• Pigmentation of nipple and areolar – persists after pregnancy
• Secondary areola may appear in dark-skinned women
• Montgomery’s tubercles become prominent
• Small quantity of clear colostrum can be expressed in latter half of pregnancy
The increase in glucocorticoids that occurs in association with the raised levels of free placental corticotrophin releasing hormone prior to the onset of labour (see Chapter 13) is also important for the breast secretory activity and milk synthesis and secretion (Casey and Plaut, 2007). Glucocorticoids play a significant role in the formation of cellular components such as rough endoplasmic reticulum and tight junctions which are required for milk synthesis and secretion. They are also involved in the regulation of milk protein gene expression and the maintenance of secretory cell differentiation and lactation by preventing the second phase of involution.
Mammary differentiation and milk secretion are coordinated by the endocrine system and involve three categories of hormones: reproductive hormones which change during reproductive development and affect mammary gland development and coordinate milk delivery; metabolic hormones which regulate metabolic responses to nutrient intake or stress; and mammary hormones produced by the lactating mammary gland (Neville et al., 2002). Lactation can be considered as two phases: lactogenesis, the initiation of lactation; and galactopoiesis (sometimes referred to as lactogenesis stage 3), the maintenance of milk secretion. Lactogenesis itself has two stages. Stage 1 is the enzymatic and cellular differentiation of the alveolar cells, which result in colostrum formation, and uptake of immunoglobulins prior to parturition but very little milk synthesis and secretion. Lactogenesis stage 2 is the onset of copious secretion of all milk components about 2–4 days after parturition following the progesterone withdrawal at parturition and concurrent stimulation by prolactin and cortisol. Lactogenesis is normally robust but may be delayed with stressful deliveries and in poorly controlled diabetes (Neville and Morton, 2001) and in obese women, probably because adipose tissue abrogates the decrease in progesterone concentration (Rasmussen et al., 2001). Maternal obesity is associated with a lower breastfeeding rate but not solely related to physiological issues; obesity affects breastfeeding intention, initiation and duration (Amir and Donath, 2007). Other factors that may delay lactogenesis are increasing maternal age, infant birthweight (over 3.6 kg), use of formula milk (especially more than 60mL in the first 48 h of life), lower maternal educational status, low Apgar scores and caesarean section (Nommsen-Rivers et al., 2009), Once lactation is commenced, it is maintained by the removal of milk which is orchestrated by prolactin, which stimulates production of milk, and oxytocin, which is involved in milk ejection (Table 16.1, Fig. 16.4). Maternal perception of inadequate milk supply may influence mothers to augment breast feeding with formula milk and thus interfere with the establishment of lactogenesis and galactopoiesis (Huang et al., 2009). Women at risk of lactational failure will need careful support and encouragement in the initiation of breast feeding. Augmentation with formula milk should be discouraged unless there are medical indications and women whose babies suckle frequently must be reassured that this does not mean their milk supply is inadequate but is an important component in establishing lactation.
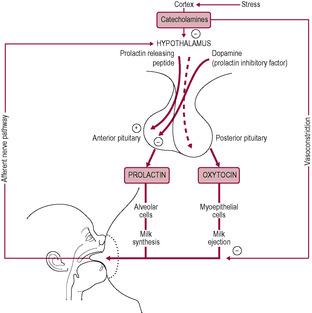
Suckling results in the firing of afferent impulses via the anterolateral columns of the spinal cord to the brain stem and hypothalamus. The hypothalamus subsequently decreases release of dopamine (formerly described as prolactin inhibitory factor) into the portal circulation to the pituitary gland. It was postulated that a dopamine-stimulating factor existed but, although several hormones positively modulate prolactin secretion, control is largely by the lifting of the tonic inhibition from dopamine. The abrogation of thedopamine inhibition stimulates the release of prolactin from the cells of the anterior pituitary. Secretion of prolactin is modified by oestrogen and thyroid-stimulating hormone (TSH). Studies in rats have demonstrated that vasoactive intestinal peptide (VIP), released from the pituitary gland, is an extremely potent prolactin-releasing factor and affects mammary blood flow. The number of signals affecting prolactin release indicates a complex neuroendocrine axis (Ben-Jonathan et al., 1991). β-Endorphin and melanocyte-stimulating hormone (MSH), which are co-released from the intermediate lobe of the pituitary gland, also seem to have a role. β-Endorphin blocks dopamine inhibition of prolactin and MSH stimulates the release of prolactin by lowering the threshold of the lactotrophs (Porter et al., 1994).
Levels of prolactin begin to rise within 10 min of suckling, peak about 30 min after initial stimulation and then progressively fall back to basal levels within a further 3 h. This delay in prolactin secretion following suckling led to the concept that the rise in prolactin was the ‘order for the next meal’. Areolar stimulation is essential for prolactin release; negative pressure alone is not adequate and denervation of the nipple prevents prolactin release in response to nipple stimulation.
Prolactin levels fall abruptly about 2 h before delivery then dramatically rebound. These fluctuations in prolactin level probably relate to changing oestrogen concentrations. The level of prolactin seems to be important in establishing lactation but levels are much diminished after 6 weeks at a rate dependent on suckling frequency and duration (Johnston and Amico, 1986). The peak prolactin levels in response to suckling also fall progressively.
Prolactin has a pulsatile release. A diurnal rhythm of prolactin secretion is apparent, with higher circulating levels during sleep. The exact quantitative relationship between prolactin levels and milk production is not clear. In the early puerperium bromocriptine, a dopamine D2 receptor agonist causes a fall in prolactin levels and abolishes milk secretion. Dopamine-receptor blockers (such as metoclopramide, haloperidol, domperidone and sulpiride) increase prolactin levels and milk production. The use of drugs which stimulate prolactin should be the last resort after excluding all the other factors which might negatively affect milk supply such as checking the positioning of the infant. All the drugs are a risk of side effects. Domperidone is usually preferred because it has a lower risk of toxicity as it crosses into the breast milk and brain to a lesser extent (it has a large molecular weight, is less soluble and is usually bound to proteins). Dopamine binds to receptors on the pituitary and is internalized resulting in the increased breakdown of prolactin within the secretory granules. However, women who have had pituitary surgery and have prolactin levels just above non-pregnant level can breastfeed. The evidence seems to suggest that a threshold prolactin level is required but then there is no correlation between prolactin level and milk production (Howie et al., 1980).
Prolactin binds to receptors on the secretory alveolar (acinar) cells acting at several sites to increase synthesis of several components of the milk including casein, lactalbumin and fatty acids. During pregnancy, secretory alveolar cells proliferate and acquire the characteristics of highly active secretory cells including numerous mitochondria, an extensive endoplasmic reticulum, well-developed Golgi apparatus and many secretory vesicles. Early suckling is important to stimulate prolactin to ensure that milk production is optimal and sustained. Infrequent and/or poor suckling in the early postnatal period may significantly reduce the optimal long term milk production as less prolactin receptor complexes are formed so stimulation of the acinar cells is sub-optimal and the potential for milk production is reduced (Manuel et al., 2007). It is important to support and reassure mothers whose infants frequently suckle in the early postnatal period to promote prolactin release and establish long term breast feeding (Case Study 16.1). Introduction of formula feeds in the belief that the infant is hungry will interfere with the establishment of lactation. In the first few hours of birth, spontaneous suckling may be facilitated by maternal–neonatal skin-to-skin contact (Bramson et al., 2010).
Almost immediately following his birth, Zara held Zak in her arms; he was naked and in direct contact with Zara’s skin and he instinctively latched onto the breast and started feeding before the cord was cut. Zak fed on the breast for over 45 min before falling asleep.
• What are the advantages of offering the baby the breast as soon as possible after delivery for both mother and baby?
• What can the midwife do to encourage Zak to spontaneously suckle at the breast with future feeding?
• What factors could have a negative influence on the early establishment of breastfeeding and how can the midwife optimize breastfeeding?
As well as the dopamine-antagonist drugs, there are a number of herbal galactogogues which have been traditionally used to promote milk production; these include fenugreek seeds, fennel, brewer’s yeast, alfalfa, asparagus, rescue remedy and ignatia 6x (Gabay, 2002). There is a lack of scientific evidence for the effectiveness of these herbal galactogogues. Smoking has been shown to reduce prolactin production (Andersen et al., 1984); in addition, psychosocial factors tend to result in lower rates of breastfeeding in women who smoke (Amir and Donath, 2003).
The secretory cells of the alveoli (Fig. 16.3) synthesize or extract the components of milk, which are secreted into the alveolar lumen. The cells are joined near their apical surface by adherins and tight junctions. The apical plasma membrane has a smooth surface with few microvilli, in contrast to the tightly folded basal membrane, which facilitates uptake of substrates such as amino acids, glucose, acetate and fats from the extracellular space. Proteins, fats and lactose are synthesized in the cell and packaged into vesicles. The vesicles move to the apex of the cell where exocytosis takes place.
(Reproduced with permission from Pond, 1992.)
The composition of the maternal diet can influence the components of breast milk, especially those passing from blood to milk with little modification by the alveolar cell, such as lipids. The alveolar cells have a unique mechanism for lipid secretion whereby microlipid droplets coalesce forming progressively larger lipid droplets that are eventually enclosed by a specialized milk fat droplet membrane prior to secretion (McManaman and Neville, 2003). Aqueous solutes including the proteins, oligosaccharides, lactose, citrate, phosphate and calcium are secreted into the milk by exocytosis after being packaged into secretory vesicles by the Golgi apparatus. Macromolecular substances derived from maternal serum, such as serum proteins (IgA, albumin and transferrin), endocrine hormones (insulin, prolactin and oestrogen), cytokines and lipoprotein lipase, are transported by a transcytosis pathway. Various membrane transport pathways transfer small molecules and ions such as glucose, amino acids and water. Large blood cells and serum follow a paracellular route, squeezing between the alveolar cells.
Oxytocin levels control the milk ejection reflex, which is responsible for the transfer of the milk from the breast to the baby. Oxytocin stimulates the myoepithelial cells so the alveolar sacs are compressed, increasing the pressure, and the ducts shorten and widen. Although secretion of oxytocin is under a similar neuroendocrine reflex to prolactin, it is physiologically independent. Oxytocin synthesis in the hypothalamus, and its release from the posterior lobe of the pituitary gland, is increased in response to handling the baby, hearing cries or thinking about feeding as well as by tactile stimulation at the nipple. Oxytocin is released in short-lived bursts of less than a minute immediately in response to stimuli. Frequently, the largest response is to the baby crying before feeding so maximum release of oxytocin may occur before suckling even starts. Between feeds, isolated pulses of oxytocin are released (McNeilly, 2001) possibly in response to other babies’ cries or fleeting images of the baby. Unlike prolactin secretion, the milk ejection reflex can be conditioned, as demonstrated by dairy farmers who clang their buckets to stimulate oxytocin and a good milk yield. Similarly, a baby’s cry can often trigger oxytocin secretion, which is why the practice of babies ‘rooming-in’ with their mothers (sleeping close to their mother’s bed) is often associated with successful breastfeeding.
The milk ejection reflex is very sensitive to inhibition by physical and psychological stresses such as pain and discomfort, anxiety, emotional swings, tiredness, embarrassment, worry and alcohol. Women who have had long labours, high levels of intervention and traumatic deliveries may be particularly at risk of impaired oxytocin production. In addition, the use of oxytocin to augment labour may reduce endogenous oxytocin levels in the early postnatal period and also affect the milk ejection reflex (Jonas et al., 2009). The limbic system, which coordinates the body’s responses to emotions, is involved in oxytocin release. The likely mechanism is catecholamine inhibition of oxytocin release and adrenergic vasoconstriction of mammary blood vessels limiting access of the oxytocin to the myoepithelial cells. Women experiencing problems in establishing milk flow are often helped by covering their breasts with warm flannels which appears to aid blood flow and oxytocin access. Women may be embarrassed by exposing their breasts or being touched by practitioners helping them to establish breastfeeding and so a ‘hands-off’ approach is best adopted whilst focusing on maintaining privacy and dignity and minimizing inhibition. Stress reducing interventions such as relaxation therapies and skin-to-skin contact have been shown to improve lactation performance (Lau, 2001).
Surprisingly, denervation of mammary glands in experimental animals appears to have little effect on milk production (Williams et al., 1993). This suggests that the afferent nerve pathway may not be as important as the interactions of neurotransmitters. Transmitters that have been implicated in the control of the milk ejection reflex include noradrenaline, β-endorphin, serotonin and dopamine. As with control of prolactin secretion, the number of factors influencing oxytocin secretion suggests that the pathway is much more complicated than originally thought. Stimulation of the female reproductive tract, especially the vagina and cervix, increases oxytocin release so milk may be ejected from the breasts during coitus.
Oxytocin binds to specific receptors on the myoepithelial cells around the milk-secreting cells and to longitudinal cells in the duct walls. Contraction of the myoepithelial cells results in milk being expelled into the ducts, which shorten as the longitudinal cells contract. Oxytocin-induced contraction generates pressure waves within the breasts and is responsible for prickly sensations associated with breastfeeding. When the milk ejection reflex is well established, milk may be spontaneously ejected from both breasts.
Oxytocin pulses increase in amplitude during labour and are involved in the positive-feedback amplification in labour (see Chapter 1). Oxytocin is associated with changes in maternal behaviour and increased alertness at delivery. The pulses of oxytocin induced by feeding have an effect on the uterus, stimulating uterine contractions and involution. Multiparous women tend to feel these contractions or ‘after-pains’ with increased intensity. Women who do not want to breastfeed may find the physiological changes in the breasts at delivery uncomfortable; various techniques are recommended to inhibit lactation (Box 16.3).
Box 16.3
• Bromocriptine (dopamine agonist) (use with great caution)
• Treatment with sex steroids to antagonize prolactin effects
• Breast-binding
• Application of ice-packs
In feeding from the breast, the baby takes the whole nipple into its mouth and places its tongue under the adjacent areola. When the baby’s tongue moves down, ducts in the nipple fill with milk which is expelled when the tongue moves upwards. The milk is expressed from the nipple and sucking aids the process.
Babies exhibit two distinct patterns of suckling (Turgeon-O’Brien et al., 1996). Nutritive suckling is a continuous stream of strong slow sucks, which efficiently allows milk transfer. This occurs predominantly in theearly part of the feed. Non-nutritive suckling increasingly replaces nutritive suckling during the progression of the feed. It is characterized by alternation of rapid shallow bursts of suckling and rests. It is thought that patterns of thumb sucking may reflect these two conducts. Breastfed babies have two distinct rhythms of thumb sucking and tend to put more of the root of the thumb into their mouths. Although non-nutritive suckling is associated with a decreased transfer of milk, it is still very effective in stimulating prolactin release and so may be important in successful lactation. It has been suggested that odours from volatile compounds in the secretions from the Montgomery’s tubercles may promote positive feeding behaviour such as rooting and contribute to the establishment of effective milk production and transfer (Doucet et al., 2009).
The amount of milk produced is extremely variable; that mothers can feed multiple babies and produce additional milk for banking or storage suggests that the mammary synthetic capacity exceeds the normal requirement of single infants. A demand-fed baby consumes irregular quantities of milk at irregular times. The suggestion that the baby determines milk yield by local control is supported by the strong correlation between degree of breast emptying and rate of milk synthesis. Some women feed exclusively from one breast (and not at all from the other). The autocrine factor capable of overriding the central hormone control, feedback inhibitor of lactation (FIL), was first identified in goats (Wilde et al., 1995). This protein inhibitory factor has also been found in the whey fraction of human milk. It is secreted from the alveolar cells and accumulates in milk. The factor inhibits secretion of lactose, probably by blocking the action of prolactin, and therefore provides the mechanism to adjust supply to demand. When milk is not removed from the breast, the concentration of the factor increases and blocks the action of prolactin thus reducing the rate of milk synthesis. It helps to explain why maternal dietary intake has relatively little influence on the amount of milk produced. Women in traditional societies have a much greater frequency of breastfeeding so their production of FIL is probably not enough to have an autocrine effect on lactation; milk synthesis is most likely to be influenced solely by metabolic and endocrine mechanisms (Hartmann et al., 1998). Ankyloglossia or tongue tie may interfere with the suckling reflex and the division of the tie (frenulotomy) may be effective in improving breastfeeding (Miranda et al., 2010).
After cessation of lactation, involution takes about 3 months. Milk accumulates in the alveoli and small lactiferous ducts, which causes distension and mechanical atrophy of the epithelial cells and rupture of the alveolar walls, creating large spaces. Milk secretion is therefore suppressed by local mechanical factors rather than by diminishing prolactin levels. Phagocytosis of the cells and glandular debris results in fewer and smaller lobular–acinar structures. The alveolar lumens decrease in size and may disappear. The alveolar lining changes from a single secretory layer to a non-secretory double layer. If breastfeeding is stopped suddenly, the process is more intense and painful. The breasts remain larger after lactation as the deposits of fat and connective tissue are increased. Involution after lactation is different to the structural atrophy and loss of adipose tissue occurring in postmenopausal mammary cells deprived of oestrogen.
Most problems have an identifiable physiological basis; breast milk insufficiency is frequently over-diagnosed and is usually simply resolved (Woolridge, 1996). The majority of women with apparently insufficient milk supply have unsubstantiated worries and require confidence, improved technique (especially positioning), encouragement or advice. This can be supported by physiological strategies such as electric breast pumps and pharmacological (anti-dopamine) agents. Avoiding pressure on the breasts (such as that due to wearing a tight bra or other tight clothing or sleeping prone) is important as it can negatively affect milk supply. Iatrogenic low milk supply may be attributed to excessive down-regulation of supply probably during the calibration period. It is possible that baby milk manufacturers inadvertently exploit the importance of the calibration period by offering free milk samples early in lactation. If the initial calibrated volume cannot be increased, the mother will then be unable to increase her milk supply later and will be forced to provide alternative inferior sources of milk, which are expensive and can be harmful if water supplies are contaminated, as happens in a number of developing countries.
Behavioural problems that are acquired by the baby as coping strategies to avoid aversive events may also induce a low milk supply. These problems include discomfort during positioning at the breast and problems with breathing. Self-limitation of intake and lack of persistence may account for the condition described as ‘contented underfed babies’ (Woolridge, 1996). Pathophysiological failure is rare and probably affects less than 2% of women with apparent milk insufficiency. Rare causes include mammary hypoplasia, or absence of normal breast development at puberty or in pregnancy. Retained placental products affect lactation reversibly but Sheehan’s syndrome, necrosis of the anterior pituitary due to acute hypovolaemic shock, as in antepartum haemorrhage (e.g. due to placental abruption) or postpartum haemorrhage, is more serious.
Many drugs are secreted into breast milk, but the data on the effects of specific drugs on the breastfed infant is often not available. Of particular concern are those drugs with central nervous system (CNS) activity as the postnatal development of the infant’s nervous system is vulnerable. The benefits of maternal treatment and the advantages of breastfeeding have to be balanced against the risk of exposure of the neonate to the medication. Passive diffusion of the unbound, unionized form of the drug into the breast milk is the major mechanism of transfer. Therefore, it is affected by maternal compartmentalization and molecular properties and the composition of the breast milk (McManaman and Neville, 2003). Assessment of adverse drug reactions in infants is difficult. Drugs that are minimally excreted into the breast milk, are metabolized quickly by the neonate and are not associated with adverse effects are obviously the preferred choice.
Socially used drugs such as alcohol (Mennella and Beauchamp, 1991), nicotine and illicit drugs (heroin and cocaine) (Golding, 1997) also cross into the milk. How much these affect the baby is not clear. Women who smoke are less likely to want to breastfeed, or initiate breastfeeding, and more likely to breastfeed for a shorter duration (Amir and Donath, 2003). Drug metabolism and elimination by the neonate is often limited, so exposure to apparently low doses of the drug in milk can have a cumulative effect, particularly in premature babies and those who have prolonged exposure. Drugs tend not to accumulate in the milk but have a bidirectional transfer. Therefore, the amount of drug received by the infant will be reduced if the mother takes the drug immediately after a feed so the baby does not feed when the drug is at peak concentration in the maternal plasma and milk. Production of breast milk is also a method of excretion and contains drugs, viruses, food additives, chemical contamination (such as lead), volatile solvents, pesticides and radioactivity. Chemical residues of pollutants are detected in most human milk throughout the world. Heavy metals are of concern because of the susceptibility of the infant’s nervous system. Mammals do not have a mechanism to excrete pesticide residues such as polychlorinated biphenyls and 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT). However, the residues do cross the blood–breast barrier so lactation is the only way to reduce the body load. The burden of persistent organic pollutants is then transferred to the breastfed infant (Nickerson, 2006). Usually, breastfeeding is not contraindicated but a slow and steady rate of maternal weight loss during lactation is important to limit the mobilization of maternal fat and release of the environmental contaminants which can then partition in the breast milk.
Women often cease breastfeeding prematurely in the first 6 months after birth because they experience breastfeeding related problems such as pain, cracked nipples, milk stasis and mastitis (Abou-Dakn et al., 2009). These conditions may occur in isolation or may be compounded by anxiety about milk sufficiency. It is estimated that a significant proportion of breastfeeding women will experience some problem in the first few days of feeding. Women experiencing breastfeeding problems are likely to have a higher level of psychological stress. Although lactation may be protective against stress in the short-term, longer lasting psychological stress may negatively affect the endocrine, immune and nervous systems (Wöckel et al., 2010).
Maternal viruses may enter the milk. Vertical transmission and subsequent infection of the infant via breast milk have been confirmed for human immunodeficiency virus (HIV), tuberculosis (TB), cytomegalovirus (CMV) and hepatitis B. It is probably inadvisable for mothers with TB to breastfeed as the infection tends to be reactivated by maternal tiredness and stress (Box 16.4). However, advice for HIV-positive mothers is unclear. The immunological properties of breast milk are probably important in protecting against illnesses that accelerate the development of AIDS (acquired immune deficiency syndrome), particularly in areas of the world where it is endemic (Van de Perre, 1995). The likelihood of HIV transmission is affected by maternal viral load, the volume of the milk consumed, the duration of breastfeeding, inflammation of the breast (e.g. caused by cracked nipples), the presence of oral thrush and the introduction of formula milk (Warner and Sapsford, 2004). (Note that several medications used in HIV prophylaxis can transfer to the breast milk and have potentially serious side effects, including anaemia, seizures, hepatitis and feeding difficulties.) The WHO recommends exclusive breastfeeding for the first 6 months of life (and breastfeeding with complementary feeding for the first 2 years of life). The rationale for this is the protective effect of breastfeeding on infection rate. However, in developed countries where the water supply is clean and good quality, alternatives to breast milk are feasible and affordable, it may be preferable for mothers with serious infections not to breastfeed to prevent vertical transmission. However, it should be noted that whatever method is chosen, it should be exclusive as mixed feeding is thought to increase the risk of transmission of HIV.
Box 16.4
• Maternal illness
• Maternal drug consumption
• Congenital abnormalities, for example, cleft palate
• HIV-positive – controversial
• TB infection (depending on strain and treatment)
Case study 16.2 is an example of concerns about breastfeeding.
Elma expressed concerns about breastfeeding throughout her pregnancy. She complained that the midwives running the antenatal classes were biased towards breastfeeding and that bottle-feeding was just as good. Elma described her own family as an example; she is the oldest of five children all of whom were bottle-fed by her mother and were well and healthy. Two days after delivery, Elma experienced breast discomfort and tentatively asked a midwife whether it was too late to try breastfeeding.
• How would you as the midwife explain and encourage Elma to breastfeed throughout the antenatal period?
• What are the health advantages for mothers and their babies who choose to breast feed? Do you think women should be informed of the higher risks of infection for the baby if the mother chooses to feed with formula milk? If so, remember it is not just because of the need to ensure sterilization of feeding equipment but formula milk lacks many of the components of breast milk that actively reduce infection in the newborn.
• What factors would increase her chances of being successful in breastfeeding?
• What support would she require from the midwives?
• Would breastfeeding or anything else help to relieve the breast discomfort?
Breast milk is also important to the infant because suppression of fertility is an advantage. An adequate birth interval is important for both maternal and child health. Lactational amenorrhoea may last from 2 months to 4 years. It is particularly important in developing countries where breastfeeding prevents more pregnancies than all the other methods of contraception put together. The variability in duration of suppressed fertility seems to be related to a number of factors; the most important seems to be frequency of suckling. At the end of pregnancy, levels of gonadotrophins are very low because high levels of oestrogen continue to impose negative feedback. At delivery, the placental hormones begin to disappear, at different rates depending on their half-life. hPL disappears from the plasma within hours. Oestrogen and progesterone levels fall to pre-pregnant levels within a week of the placental loss (Hytten, 1995). Levels of human chorionic gonadotrophin (hCG) are negligible about 3 weeks after delivery. There is a gradual recovery in the ovarian–pituitary axis over the first 4 months after delivery; this recovery is delayed by regular suckling.
In non-lactating women, body temperature measurements and the first menstrual bleeding suggest that the earliest ovulation may occur at 4 weeks after delivery but is usually delayed until 8–10 weeks (McNeilly, 2001); most women have resumed normal menstrual patterns by 15 weeks. The first menstrual cycle is often anovulatory or associated with an inadequate luteal phase. Most women ovulate by the third cycle. Fifty percent of non-lactating women who do not use contraception conceive within 6–7 months.
Menstruation and ovulation return more slowly in a lactating woman. Ovarian activity usually returns before the end of lactational amenorrhoea. Therefore, menstruation is a poor indicator of fertility; conception can occur before the resumption of menstrual cycles. Neither ovulation nor menstruation normally occurs within 6 weeks, but about half of all contraceptive-unprotected breastfeeding mothers conceive within 9 months of lactation, 1–10% during lactational amenorrhoea. Between 30% and 70% of first cycles are ovulatory; the longer the period of lactational amenorrhoea, the more likely the woman is to ovulate prior to the first menstruation.
The precise mechanisms involved in lactational amenorrhoea are not clear. High prolactin levels abolish the pulsatile luteinizing hormone (LH) secretion and decrease the pituitary response to gonadotrophin-releasing hormone. The mid-cycle positive feedback in response to oestrogen is absent. The sensitivity to negative feedback is enhanced and that to positive feedback is decreased. So, even if enough LH and follicle-stimulating hormone (FSH) are present to stimulate follicular development, the inhibitory effect of oestrogen results in an inadequate luteal phase. Prolactin is inhibitory at the level of the ovary, blocking the effects of LH and FSH. It also has a direct effect on the brain, possibly affecting libido.
As prolactin secretion has a pulsatile rhythm with larger amounts being released at night, the frequency of stimulation by suckling and the night-time feeds are particularly important in maintaining prolactin levels high enough to suppress fertility (McNeilly, 2001). The duration and number of feeds are important because the prolactin levels are augmented before they return completely to the basal secretory level. Prolonged amenorrhoea is associated with maternal malnutrition (Rogers, 1997). Poor nutrition is associated with suppression of fertility in non-lactating women. The extra nutrient requirement for milk production can increase the degree of maternal malnutrition. Also, although women receiving less than optimal nutrition can breastfeed their babies adequately, they secrete milk more slowly so the infants feed more often and for longer which raises their circulating prolactin levels.
Maternal commitment to reproduction is more than pregnancy; it involves the establishment of lactation and appropriate maternal behaviour (Grattan, 2002). The demands of the parents and offspring during lactation may conflict. It is suggested that parents will tend to maximize the survival of their young but not to the extent that would limit investment in other offspring, including those as yet unborn (Peaker, 1989). This theory means that, although mothers will try to recoup the investment of pregnancy by favouring the offspring’s survival, should this cost compromise their future reproductive ability there are definite advantages in discontinuing this investment in favour of a more favourable future offspring. There may be genetic components affecting the time course of lactation or the upper limit of milk production. The rate of milk secretion and duration of lactation vary with nutritional state. Some mammals respond to decreased food supply in ways that favour the succeeding pregnancies, such as killing some or all of the litter. Species with long gestation and long-term commitment to the offspring, such as humans, tend to favour the well being of live offspring.
Behavioural changes include preparatory behaviour such as nest building and increased aggression. Care and protection are associated with lactation, particularly the maternal level of oxytocin (Gordon et al., 2010). These behavioural patterns are associated with the progressive independence of the young. In humans, this behaviour is more difficult to observe than in other species.
The nutritional status of the mother may affect feeding and interaction with her infant (Britton, 1993). The effect of maternal malnutrition can affect infant development, depending on its duration and the timing. Infants malnourished in utero may have decreased capacity to respond to appropriate cues and therefore an increased likelihood of social and further nutritional deprivation. Malnourished infants have poorer muscle tone, increased lethargy, irritability and frequency of illness, decreased attention and responsiveness and altered sleep–wake states. Malnourished mothers experience more fatigue, which can affect their own sensitivity to cues from the baby, such as responses to stress and attention–behavioural patterns.
Human growth rate is much slower than that of other animals. Neurological development is relatively late and the duration of human lactation is longer. During lactation, daily nutrient input and reserves laid down in pregnancy are juggled. Milk output is largely independent of the mother’s ethnic origin and nutritional status. A balanced diet in lactation appears to favour the health of the mother. However, it should be noted that substrates required for milk synthesis are not flexible. Mammary glands are not able to synthesize essential amino acids or long-chain polyunsaturated fatty acids (LC-PUFAs); they also require non-essential amino acids for protein synthesis and glucose or glucose precursors for lactose and oligosaccharide synthesis. These have to be provided from the maternal diet or from maternal body reserves. Indeed, it has been suggested that the maternal brain may act as a reservoir of PUFAs for both fetal and neonatal brain requirements (Dewar and Psych, 2004) and that increased transfer of nutrients to the developing brain during pregnancy and lactation might contribute to postnatal depression.
The energy output of milk is a significant proportion of the total energy output of the lactating woman; it is suggested that peak lactation requires an increase of energy intake of about 25%. In dairy animals, the level of food intake strongly correlates with milk yield. In a number of species, lactation results in a significant increase in size and complexity (such as villus size) of the maternal intestine (Hammond, 1997), but it is obviously not possible to study any such adaptive changes in lactating women. It may not be valid to apply knowledge of nutrition and physiology of dairy animals, which are completely milked twice a day, to mammals that suckle their young according to natural patterns of behaviour. Anthropological studies on human hunter–gatherer communities suggest that babies feed every half hour at 2 weeks and every 4 h at 4 months. The characteristics of mammalian milk relate directly to the interaction between the mother and child. Marsupials and animals that bear their young during hibernation are always present and produce milk that is dilute and has a low fat content. In contrast, in animals where the mother nurses her young at widely spaced intervals, for instance a hunting lioness, the milk is very concentrated and high in fat. Human milk has most resemblance to the former; it is dilute with a low fat content suggesting that humans have evolved as a species where the young have unlimited access to milk and there is high attentiveness shown by a constantly present mother (Prentice and Prentice, 1995). The stress of human lactation is relatively low compared with species with faster growing or multiple young, but this is countered by the high cost of maintaining a dependent infant for a prolonged period. The high level of maternal investment in pregnancy and slow reproductive cycle mean that humans are committed to sustain a conception.
There is a discrepancy between the theoretical calculated energy requirement for milk production and the actual intake of lactating women, even taking into account the fat reserves laid down in pregnancy. Current recommendations are that an exclusively breastfeeding woman requires an additional 2700 kJ (650 kcal) per day and an increase of 20 g of protein per day (Dewey, 1997). However, it is thought that about 600 kJ (150 kcal) per day will be provided by the maternal fat stores for the first 6 months so the net increment needed is 2100 kJ (500 kcal) per day. In practice, these requirements are much higher than the observed intakes in successfully lactating women even when offered unlimited access to food. Lactational performance is particularly resilient in humans as demonstrated by the efficiency of lactation in undernourished and impoverished communities. In animals, a decrease in non-shivering thermogenesis (inhibition of brown-fat heat generation) (NST) and therefore the provision of extra energy for milk production are suggested to account for this difference. The mechanism in humans is not thought to be mediated by changes in NST but the lactating woman has increased sensitivity to insulin (Illingworth et al., 1986). This energy-sparing effect and efficient energy utilization of lactating women have a particularly big implication in developing countries.
Increased incidence of obesity in Western societies is of concern with about a third of women having a body mass index (BMI) greater than 25 kg/m2. Pregnancy is a risk factor for the development of obesity; it is suggested that postpartum weight loss may not be inevitable and gestational weight gain may not be lost postpartum (Chin et al., 2010). Possibly, the changes in energy metabolism associated with pregnancy and lactation may remain after weaning. If so, lactation could contribute to the problem. Different species of mammal lay down body fat during pregnancy to different degrees. In lactation, mammals rely on the deposited fat to different extents. Whales and seals, for instance, rely entirely on body fat and protein reserves to sustain lactation whereas dairy cows and laboratory rats are very dependent on increased intake to provide energy for milk yield. Pregnant women deposit fat and have a changed hormonal environment. The reported studies tend to conflict and do not show significant differences in weight loss with different patterns of infant feeding. However, interpretation of the studies is confused by confounding factors such as different duration and extent of feeding and the increased tendency of women who are not breastfeeding to reduce their weight deliberately. There is also a large variation in the energy content of the milk produced (see below). Lactating women produce adequate-to-abundant quantities of milk of sufficient quality to promote growth of healthy infants, even when maternal nutrition is not adequate. Whereas the health of the breastfeeding infant is apparently protected should maternal nutrition be compromised, it is probably at the cost of depletion of maternal nutrient stores and potential effects on subsequent pregnancies. Deliberate weight loss in well-nourished healthy breastfeeding woman has no effect on the yield or composition of breast milk. However, it is recommended that weight loss should not be more than 1–2 kg per month (Institute of Medicine, 2002). Although reduction in energy intake does not affect milk synthesis, lower intakes of food mean that micronutrient intake, particularly calcium and vitamin D, might be compromised (Lovelady et al., 2006). As energy intake falls, the likelihood of a number of nutrients failing to reach the recommended intake progressively increases. In order of vulnerability, the nutrients most likely to be affected are calcium, zinc, magnesium, thiamin, vitamin B6, vitamin E, riboflavin, folate, phosphorus and iron (Lawrence, 2010). Overweight lactating women are advised to restrict their energy intake by decreasing consumption of foods high in simple sugars and fat and by increasing their intake of calcium-rich foods, vegetables and fruit.
Both pregnancy and lactation present a tremendous challenge to maternal calcium status (Prentice, 2000). The fetal skeleton requires 5 mmol of calcium per day. The lactational drain of calcium is greater; average daily production of 800mL milk contains 6.25 mmol of calcium. It is estimated that about 10% of total maternal calcium stores (about 105 g) is transferred to the fetus/neonate, predominantly duration lactation (Wysolmerski, 2002). One or more of the following can meet demand for calcium: increased dietary calcium, increased absorption, decreased excretion or increased bone demineralization (net loss of bone). In the third trimester, absorption of calcium increases together with a modest increase in bone resorption. This increase in calcium absorption appears to be independent of vitamin D status (Fudge and Kovacs, 2010). Calcium demands of lactation are met by an increase in the rate of bone resorption and a decrease in renal calcium excretion which are speculated to have evolved from the adaptations in bone and mineral metabolism that supply calcium for egg production in lower vertebrates (Wysolmerski, 2002). The oestrogen level during lactation is relatively low so the bone mass is not protected to the same extent.
Lactation is the period of most rapid bone loss in a woman’s life; there is a net drain of calcium from the body, with a selective decrease in trabecular bone. This reduction is independent of parathyroid hormone and vitamin D levels. So lactation may appear to increase future risk of osteoporosis, but risk factors shown to be associated with fractures do not necessarily include breastfeeding (Sowers, 1996). During weaning, an imbalance between bone resorption and bone formation results in a rapid and complete recovery of bone mass. The implications for birth spacing and prolonged breastfeeding are not clear. Modern practices of delaying childbearing resulting in decreased time for recovery before menopause are probably countered by the cumulative effect of having fewer children. Prolonged lactational amenorrhoea may help to restore maternal iron status.
The mammary gland homeostatically controls milk concentration of essential nutrients; levels of major minerals including calcium, sodium, potassium, phosphorus and magnesium are not affected by the diet. The mammary gland can adapt to maternal deficiency (or excess) of iron, zinc and copper (Lönnerdal, 2007) suggesting there are active transport mechanisms for these nutrients in the mammary gland. When milk production falls during weaning, milk iron levels decrease and milk zinc levels increase. However, maternal intakes of iodine and selenium do affect levels in milk. As iodine is so important for fetal and neonatal brain and nervous system development and many women do not have an optimal intake of iodine (partly because salt use is decreasing and fewer iodine-containing cleaning agents are used in the dairy industry), iodine supplements are widely recommended for pregnant and lactating women and for women considering pregnancy (Zimmermann, 2009).
The volume of milk produced is robust; only very severe dehydration and extreme malnutrition affect the volume of milk produced. There is no evidence that increasing fluid intake increases the volume of milk produced or that reducing fluid intake prevents engorgement. When fluids are restricted, urine output decreases and the woman is at risk of dehydration. Breastfeeding women should be advised to drink when they are thirsty and to be aware that they will need more fluid than normal.
Human milk optimally fulfils the nutritional requirements of the human neonate. It has a unique composition that is particularly suitable for the rapid growth and development of the infant born with immature digestive, renal and hepatic systems. Unique features of human milk are able to compensate for the underdeveloped neonatal capabilities. Human milk contains not only the macronutrients, vitamins and minerals but also non-nutrient growth factors, hormones and protective factors.
There are at least 100 components of human milk, including substances yet to be identified and their roles elucidated. In the Koran, breast milk is described as ‘white blood’. This is a particularly apt description, because the early milk has more white blood cells than blood itself. Milk is a solution in which other substances are dissolved, emulsified or colloidally dispersed. The value of breast milk is undisputed; rarely should breastfeeding be discouraged.
Both the volume and composition of human milk are extremely variable. Some of this variability is genetic (any genetic mutation leading to inadequate mammary development in humans would no longer be eliminated as alternatives to human milk are available). Postponing childbearing until long after sexual maturity has an effect on breast development as advancing age causes some atrophy of the mammary tissue (Hytten, 1995). However, there is little relationship between the size of the breast and milk output.
The unique characteristic of humans is the large complex brain, which undergoes much development in the first 2 years of life. Human milk provides levels of lactose, cysteine, cholesterol and thromboplastin, which are required for CNS tissue synthesis. However, as breast milk provides a model of optimum nutrition, analysis of its composition has allowed good substitutes to be produced as formula feeds. Infant formula milk will never completely mimic human milk, however, as the quality of the nutrients is not reproducible and the immunological aspects of the milk make it superior.
Although breast milk may be considered perfect nutrition, its composition is variable. It varies from woman to woman, from one period of lactation to another, and hourly through the day. Its composition is related to the timing of the feed, how much is produced and parameters relating to the last feed (Emmett and Rogers, 1997); it has also been suggested that maternal age, parity, health and social class affect the composition of the milk. Mothers of premature infants produce milk that has a higher concentration of some nutrients, but this probably reflects the small volumes produced for small infants. Except for vitamin and fat content, the composition is largely independent of maternal nutrition unless the mother experiences severe malnutrition. Supplementation may improve maternal health rather than affect milk composition and volume.
There are many difficulties encountered in the estimation of the volume of milk produced. Weighing either the mother or baby before and after the feed is fraught with problems. Although double-labelled water measurements have allowed more accurate estimations (Lucas et al., 1987), the variability within a feed and from feed to feed makes it very difficult to ascertain precisely the nutrient consumption of a healthy growing baby. These estimates of about 60 kcal per 100mL are lower than UK food composition tables (69 kcal per 100mL). The volume of daily milk intake by healthy infants has a wide range. Factors that influence frequency, intensity or duration of feeds will affect volume consumed. Breastfed infants appear to self-regulate their energy intake and consume more milk if it has a lower energy level. This is thought to be related to the lower incidence of obesity in individuals who were breastfed as infants (Dewey, 2003). Breastfeeding allows infants to learn self-regulation of energy intake whereas bottle-fed infants may be encouraged to finish the bottle which suppresses their autoregulatory mechanism. Breast milk may also contain appetite inhibitors and stimulators. It is suggested that different modes of infant feeding have different metabolic programming effects. Formula-fed infants tend to receive more protein which results in an increased insulin response. Insulin stimulates adipose tissue deposition (increased number and fat content of adipocytes) which is associated with weight gain and obesity. Alternatively, breastfeeding may affect leptin metabolism; the higher level of fatness in formula-fed infants may programme reduced sensitivity to leptin in later life. However, it should be noted that there is confounding by parental attributes and the family environment. Parents who choose to breastfeed tend to have a healthier lifestyle with more optimal dietary habits and higher levels of physical activity; they also exert less parental control over child-feeding practices (Dewey, 2003).
The low levels of gastric secretion and other immature digestive characteristics of the neonatal gut confer a number of immunological advantages which were described in Chapter 15 (p. 397).
In the first 3 days postdelivery, the mother produces about 2–10mL of colostrum per day. More colostrum is produced sooner if the woman has had previous pregnancies, particularly if she has lactated before. In some cultures, colostrum is thought to be old milk or ‘pus’ and is discarded rather than fed to infants.
Colostrum is transparent and is yellow from the high β-carotene content. Mature milk in contrast looks less viscous and slightly blue. Colostrum has more protein and vitamins A and K and less carbohydrate and fat than mature milk. It is easily digested and well absorbed. It has a lower energy content of 58 kcal per 100mL compared with 70 kcal per 100mL in mature milk. Levels of sodium, potassium, chloride and zinc are high in colostrum but these reflect the low volume produced rather than the infant’s requirements for a bolus dose of certain nutrients. The composition is extremely variable, which reflects its unstable secretory pattern.
Colostrum facilitates the colonization of the gut with Lactobacillus bifidus (Wharton et al., 1994). Meconium also contains growth factors for L. bifidus. Colostrum seems to have a laxative effect, stimulating the passage of meconium. The high protein content is largely due to the abundant antibodies, which protect against gastrointestinal tract infection. IgA forms 50% of the protein content of colostrum, falling to 10% by 6 months. In the first few days of life, priming and maturation of the mucosal immune system is maximal and the gut is permeable and able to absorb macromolecules; colostrum contains many immunomodulatory molecules particularly anti-inflammatory agents which help to protect the vulnerable immature gut from mucosal damage.
During the first 30 h or so, the secretion (colostrum) has a high protein:lactose ratio. In the following days, as the baby suckles more and stimulates milk production, the resulting increase in prolactin secretion stimulates production of the major whey protein α-lactalbumin, which is a specific component of the enzyme lactose synthetase and so regulates lactose production. The effect of increasing lactose production is that water is drawn into the secretion to maintain osmotic equilibrium so the volume increases thus diluting the protein content. The absolute amounts of protein secreted into the milk are maintained or increased even though the concentration falls.
Get Clinical Tree app for offline access