CHAPTER 12
Intrapartum Fetal Assessment
1 Identify techniques and methods of fetal assessment.
2 Identify baseline (BL) features, including rate, rhythm, and variability.
3 Identify, interpret, and discuss probable causes of periodic patterns of variable, late, and early decelerations and accelerations.
4 Identify, interpret, and discuss probable causes of episodic patterns of variable and prolonged decelerations and accelerations.
5 Identify, interpret, and discuss probable causes of arrhythmic and dysrhythmic fetal heart rate (FHR) patterns.
6 Assess probable fetal status.
7 Discuss strategies to document FHR events accurately and completely for the patient record.
8 Reiterate the critical values of fetal blood gases and pH, and relate these to fetal outcome.
9 State appropriate physiologic interventions for deceleration patterns and altered variability in relation to fetal outcome.
INTRODUCTION
Intrapartum fetal assessment is essential to providing critical information regarding fetal well-being and the fetal response to labor. FHR and uterine activity (UA) data can be collected by nonelectronic or electronic methods. Regardless of the method chosen to assess fetal status, the nurse is accountable for knowing and responding to auditory and electronically obtained data (Curran & Torgersen, 2006; Harmon, 2009). Nonelectronic assessment uses auscultation and palpation to assess the FHR and UA. Electronic assessment or electronic fetal monitoring (EFM) uses electronic techniques, such as tocodynamometer, ultrasound, fetal scalp electrode (FSE), or intrauterine pressure catheter (IUPC) to monitor FHR and UA. The technique provides a permanent record that can be observed and discussed instantaneously or retrospectively by professional care providers. Events that cannot be heard or measured by auscultation, such as variability, are available through EFM. Therefore, EFM is another tool available to the care provider to easily provide information that would otherwise consume many hours of care and yield less complete data. The ability to monitor using short- or long-distance telemetry influences nursing care management and patient comfort and may aid consultation and transport practices. Although there are few confirmatory data that show that the use of EFM has significantly improved outcomes, it is still used in the labor and delivery arena (Simpson, 2008a). The reader should also be aware of research in progress to refine or augment interpretation of fetal status and probable outcomes. Such methods that may become available include oxicardiotocograph, computer analysis of fetal electrocardiogram, near-infrared spectroscopy for high-risk pregnancies, lactate measurement as a replacement for pH fetal blood sampling, ST-segment analysis, and artificial intelligence to assess all fetal data and clinical events. Today, proper interpretation of FHR patterns may be the best method to reliably determine fetal status.
In 1995-1996 the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) pulled together EFM experts to discuss EFM terminology. In 1997 this group of experts published new EFM definitions that were based not only on clinical data, but also on laboratory, manufacturing, and published research. These definitions were “reaffirmed and updated at the 2008 meeting” (Lyndon, O’Brien-Abel, and Simpson, 2009, p. 104). One goal of these definitions was to allow the predictive value of fetal monitoring to be assessed more meaningfully. Another was to allow evidence-based clinical management of intrapartum fetal compromise (Macones, Hankins, Spong, Hauth, & Moore, 2008). In December 2004, the Society of Obstetricians and Gynecologists in Canada (SOGC) adopted the NICHD nomenclature as their standard in the interpretation of FHR tracings. In May 2005, the American College of Obstetricians and Gynecologists (ACOG) and the Association for Women’s Health, Obstetric, and Neonatal Nurses (AWHONN) followed suit and also adopted the NICHD nomenclature to be used in the interpretation of FHR tracings. The language used in this chapter is consistent with the most recently understood interpretations of EFM tracings in general use and is consistent with AWHONN’s current Fetal Heart Monitoring Program, revised in 2006, 2008, and 2009 to coincide with the NICHD. The reader must recognize that although the NICHD committee agreed to the FHR that was definitely evident of a fetus that was well oxygenated and agreed to the FHR that definitely needed immediate intervention, there was not consensus on FHR patterns that fell between those two extremes (Parer & Ikeda, 2007).
Regardless of the practice setting, the terminology, or even the areas of non-consensus, the process of interpreting a FHR monitoring tracing includes (a) examining the tracing for trends of FHR and UA parameters and (b) answering the question, “At the present time, what is the likely status of this fetus?” (Curran & Torgersen, 2006; Lyndon et al, 2009; Macones et al, 2008). Nurses using EFM should know the capabilities, benefits, limitations, and troubleshooting of the assessment modalities used.
CLINICAL PRACTICE
A Nonelectronic methods of FHR assessment
1. Auscultation (Curran & Torgersen, 2006; Feinstein, Sprague, & Trepanier, 2008)
(3) Verifies presence of an irregular rhythm (i.e., dysrhythmia)
(4) Detects increases and decreases from FHR BL
(5) Clarifies halving or doubling on the EFM tracing
(6) Differentiates fetal and maternal heart rates, eliminating errors related to fetal demise and EFM equipment errors
c. Benefits (Feinstein et al, 2008)
(1) Outcomes are comparable to those with EFM based on current randomized clinical trials (RCTs).
(2) Lower cesarean birth rates have been associated more with auscultation than EFM in some RCTs.
(4) Promotes the “high-touch, low-tech” approach to care
(5) Widespread application is possible.
(6) Patients have increased freedom of movement and ambulation.
(7) Allows for FHR assessment during water immersion
d. Limitations (Curran & Torgersen, 2006; Feinstein et al, 2008)
(1) May limit ability to hear FHR resulting from maternal obesity, increased amniotic fluid volume, and maternal or fetal movement
(2) Uterine tension disrupts assessment.
(3) Certain FHR characteristics associated with EFM cannot be detected (e.g., variability, types of decelerations).
(4) Some women may believe that auscultation is more intrusive or disruptive.
(5) Is not automatically documented on paper; therefore, cannot go back and review tracing
(6) Creates a potential need to increase or realign staff to meet 1:1 nurse/patient ratio
(7) Requires education, practice, and skill in auditory assessment
(3) Increases and decreases (abrupt or gradual) in BL rate
(4) Timing related to contraction
(5) Frequency for intermittent auscultation (IA) (ACOG, 2005; AWHONN, 2008; SOGC, 2007). note: These guidelines may change as new data are presented. This information is a guideline and not suggestive of or dictating an exclusive procedure or plan of action. The individual patient and her clinical situation must always be considered to ensure an individualized plan of care.
(a) AWHONN: auscultation and EFM
[i] Low risk: every 15 to 30 minutes during active phase, every 5 to 15 minutes during second stage
[ii] High risk: every 15 minutes during active phase, every 5 minutes during second stage
[i] Low risk: Latent phase. Regularly after rupture of the membranes or other clinically significant change. (note: These patients are usually at home during this time. However, if they are in the hospital, this is one recommended way to perform IA. There is just not enough evidence to recommend optimal frequency time for latent phase of labor [SOGC, 2007]).
[ii] Low risk: Auscultate every 15 to 30 minutes in active labor, every 15 minutes in second stage then every 5 minutes once pushing has started.
[iii] EFM: every 15 minutes during the active phase of labor, at least every 5 minutes during the second stage of labor
a. Detects relative uterine resting tone (Harmon, 2009)
b. Detects relative frequency, duration, and relative strength of uterine contractions (Harmon, 2009).
c. Benefits (Curran & Torgersen, 2006; Harmon, 2009; Simpson, 2008a)
(1) Noninvasive; allows for hands-on assessment or care of patient
(2) Widely used; not limited by access to equipment
(3) Patient has increased freedom of movement and ambulation
(4) Allows for touch therapy that may be reassuring to some patients
(6) Inexpensive when compared to additional devices such as an IUPC
d. Limitations (Curran & Torgersen, 2006; Harmon, 2009; Simpson, 2008a)
(1) Actual intrauterine pressures cannot be detected.
(2) Certain conditions may limit ability to palpate contractions (e.g., maternal size, large amount of adipose tissue).
(3) Subjective assessment; varies from examiner to examiner
(4) No permanent record is provided.
(5) Potential for the mother not to tolerate palpation during labor
B Electronic methods of FHR assessment
1. Doppler ultrasound for FHR assessment
a. Detects FHR BL rate, accelerations, decelerations, and variability of the FHR
b. Benefits (Curran & Torgersen, 2006; Harmon, 2009; Simpson, 2008a)
(1) Is noninvasive; easy to use
(2) Rupture of membranes or cervical dilation is not required.
(3) Creates a permanent record via hard copy, electronic disks, or microfilm process
(4) Can be observed from many locations when visual screens are used as a central display or as a bedside monitor to track all patients
(5) Can use in the intrapartum and antepartum arena.
(6) Can be used with telemetry allowing for monitoring during ambulation
c. Limitations (Curran & Torgersen, 2006; Moffatt & Feinstein, 2003; Simpson, 2008a)
(1) Signal transmissions may be influenced by maternal obesity, occiput posterior fetal position, anterior placenta, and fetal movement (e.g., weak, absent, or false signal).
(2) Maternal movement may be restricted.
(3) Maternal and fetal movement may interfere with continuous record.
(4) Artifact may artificially increase variability.
(5) Monitor may half- or double-count, especially if FHR tachycardia or bradycardia is present.
(6) May detect maternal aorta movement and trace as FHR, especially if fetus is small
(7) May detect maternal heart rate as FHR at random or in the event of fetal demise
a. Detects FHR BL rate, variability, accelerations, and decelerations
c. Benefits (Harmon, 2009; Simpson, 2008a)
(1) Provides continuous detection of FHR
(2) Accurately detects variability
(3) Maternal position change does not alter assessment ability or tracing quality.
(4) Provides a permanent record
(5) Can be observed from many locations when visual screens are used
d. Limitations (Harmon, 2009; Simpson, 2008a)
(1) The procedure is invasive.
(2) Rupture of membranes, cervical dilation, and accessible or appropriate fetal presenting part are required.
(3) Moist environment is required for detection of FHR.
(4) Potential small risk of infection, fetal hemorrhage, or fetal injury is presented.
(5) May not be accurate when fetal demise occurs; maternal heart rate may be detected and traced.
(6) Fetal dysrhythmias may not be evident if logic or electrocardiogram (ECG) button is turned on or engaged.
3. Tocodynamometer (tocotransducer) for UA assessment
a. Detects relative uterine resting tone
b. Detects relative frequency and duration of uterine contractions
c. Benefits (Curran & Torgersen, 2006; Harmon, 2009; Simpson, 2008a)
(3) Ruptured membranes or cervical dilation is not required.
(4) Creates a tracing via hard copy, electronic disks, or microfilm process for future assessment and for permanent medical record
(5) Can be used in antepartum and intrapartum arena
(6) Can be used with telemetry providing for monitoring during ambulation
d. Limitations (Curran & Torgersen, 2006; Harmon, 2009; Simpson, 2008a)
(2) Cannot detect uterine contraction intensity and resting tone
(3) May be unable to accurately detect exact uterine contraction frequency and duration in some conditions (e.g., maternal obesity or preterm labor)
(4) Location sensitive; placement may lead to false information.
(5) Sensitive to maternal or fetal motion that may be superimposed on the waveform
(6) Transducer presence or position may be uncomfortable for mother.
(7) Maternal movement and ambulation during labor may be limited.
(8) May need frequent readjustments secondary to maternal and fetal movement.
a. Detects actual uterine resting tone and frequency, duration, and strength of uterine contractions
b. Access for amniotic fluid testing (e.g., amniotic fluid sampling)
d. Benefits (Curran & Torgersen, 2006; Harmon, 2009; Simpson, 2008a)
(1) Objective; accurate assessment of uterine contraction frequency, duration, intensity, and resting tone
(2) Correlation of timing of FHR changes with UA is more accurate.
(3) Provides a tracing via hard copy, electronic disks, or microfilm process for future assessment and for permanent medical record
(4) Provides means for aspiration of amniotic fluid to assess for chorioamnionitis
(5) Provides means for amnioinfusion as intervention for oligohydramnios or thick meconium-stained amniotic fluid
(6) Solid-tipped IUPC is easily zeroed/rezeroed to atmospheric pressure.
(7) Solid-tipped IUPC design avoids pressure artifacts that may be caused by a catheter containing air, that becomes kinked, or becomes lodged against the uterine wall, as may occur with fluid-filled IUPC.
(8) Can be used to calculate Montevideo units during oxytocin infusion
(9) Assists in interpretation of late versus variable decelerations
e. Limitations (Curran & Torgersen, 2006; Moffatt & Feinstein, 2003; Simpson, 2008a)
(a) Rupture of membranes (ROM) or adequate cervical dilation required.
(c) Maternal ambulation may be limited during labor.
(d) Increased risk of uterine perforation (rupture), placental abruption or perforation, infection, or umbilical cord prolapse.
(e) IUPC reading may be different between fluid-filled and sensor-tipped catheters.
(f) May be contraindicated with infections in which ROM is discouraged to prevent maternal-fetal transmission (e.g., group B-streptococci, genital herpes, human immunodeficiency virus [HIV]).
(a) Catheter tip may become wedged against uterine wall or fetal part; might prevent production of pressure data or produce distorted or truncated waveform.
(b) Catheter tip may affect pressures, especially as related to external pressure transducer.
(c) Catheter may become obstructed with meconium, vernix, or blood.
(d) Pressure readings may be lower than solid-tipped IUPC transducer.
(e) Air may dissipate or pass through balloon at tip; may change pressure waveform or provide inaccurate pressure data (solid-tipped IUPC with pressure balloon).
FETAL FACTORS INFLUENCING THE FHR
A Parasympathetic nervous system
2. Provides variability; greatest influence on short-term variability (STV)
4. Increases with increasing gestational age
5. Effect on FHR may be exaggerated during hypoxemia.
6. Blocking (e.g., with atropine) produces increased FHR and loss of variability
1. Increases FHR and cardiac output
2. Some influence on long-term variability (LTV) in conjunction with parasympathetic nervous system.
3. Influences epinephrine and norepinephrine (epinephrine secreted in significantly smaller amounts than norepinephrine)
4. Effect on FHR may be stimulated during hypoxemia.
5. Blocking the sympathetic system, as with maternal medication, produces decrease in the BL FHR.
1. Located in carotid artery and aortic arch
2. Respond rapidly to fetal blood pressure (BP) changes; transfer information to sympathetic and parasympathetic systems
3. An increase in fetal BP produces decrease in the FHR, which decreases fetal cardiac output and BP
4. Decreases in fetal BP result in sympathetic stimulation to increase FHR.
D Chemoreceptors (Nageotte & Gilstrap, 2009; O’Brien-Abel, 2009; Simpson, 2008a)
1. Located in aortic arch and carotid artery
2. Consists of peripheral receptors in the central nervous system (CNS)
3. Responds to fetal oxygen or carbon dioxide changes and in pH levels of blood and cerebrospinal fluid; transfers information to sympathetic and parasympathetic nervous systems
4. Least-understood factor influencing FHR
5. Stimulation caused by mild increases in carbon dioxide or mild decreases in oxygen produces an increase in fetal BP and FHR; more severe changes can produce bradycardia.
1. Responsible for variations in FHR and variability in response to fetal sleep state and body movements; in fetal sleep cycles of 20 to 40 minutes, variability and reactivity decrease
2. In alert states, FHR variability and reactivity increase.
3. Integrative center for central and peripheral neural influences that produces variability and net increase or decrease in BL FHR
1. Catecholamines facilitate hemodynamic changes in response to hypoxemia and adaptational changes in neonate at birth (Lagercrantz & Slotkin, 1986; Parer, 1976, 1999).
2. Epinephrine increases FHR and blood flow to skeletal muscle.
a. Associated with initial increase in FHR (Lagercrantz & Slotkin, 1986; Parer, 1999)
b. Increases blood flow to vital organs and away from nonvital organs
c. Hemodynamic changes elevate BP and may cause parasympathetic response resulting in decreased FHR (norepinephrine cannot overcome this parasympathetic response).
a. Secreted by pituitary; increases during hypoxemia and hemorrhage
c. Produces rise in BP by increasing peripheral vascular resistance and decreasing FHR
a. Renin secreted by kidneys; increases in response to hemorrhage
(1) Secreted by kidneys; increases in response to hemorrhage and hypovolemia.
(2) Causes vasoconstriction of peripheral vascular bed resulting in maintenance of systemic arterial BP and umbilical-placental blood flow.
(3) Increased level produces marked increase in BP with initial decrease in FHR followed by increase to higher than previous FHR; produces increased cardiac output and blood flow to heart.
BASELINE CHARACTERISTICS
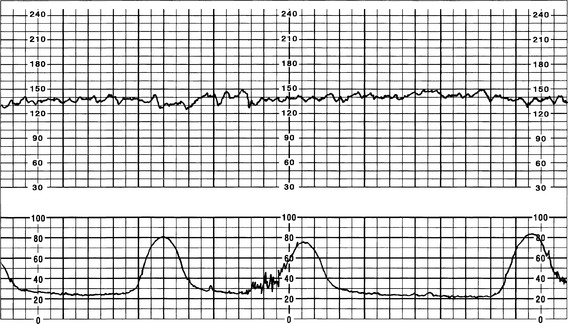
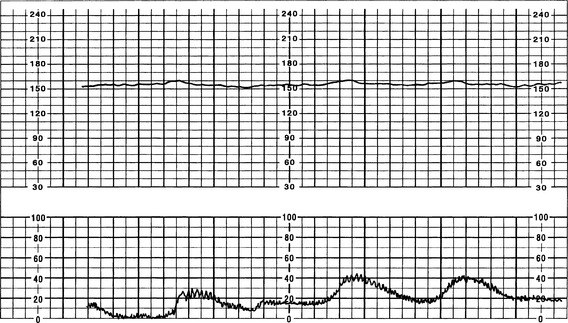
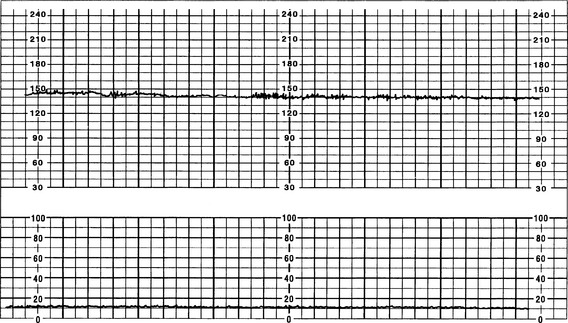
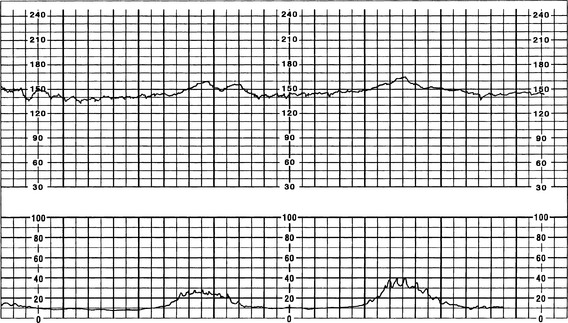
The FHR BL is assessed by determining the mean FHR rounded to increments of 5 beats per minute (bpm) during a 10-minute window. Additionally, there needs to be 2 minutes of interpretable data to determine FHR BL. The 2 minutes do not necessarily have to be continguous—they may be 2 consecutive minutes or two 1-minute segments (Curran & Torgersen, 2006; Macones et al, 2008; Nageotte & Gilstrap, 2009; Simpson, 2008a; Tucker, Miller, & Miller, 2009). Normal FHR BL ranges from 110 to 160 bpm; it is assessed when mother has no uterine contraction and excludes accelerations, decelerations, and periods of marked variability (>25 bpm). If a FHR BL cannot be determined (i.e., not enough data to interpret, marked variability present so cannot determine BL, sinusoidal pattern, etc.), the BL is interpreted as “indeterminate” (Figure 12-1).
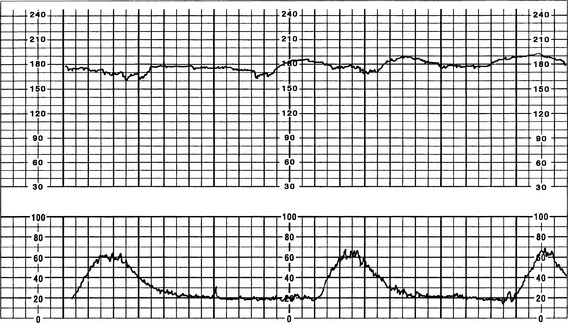
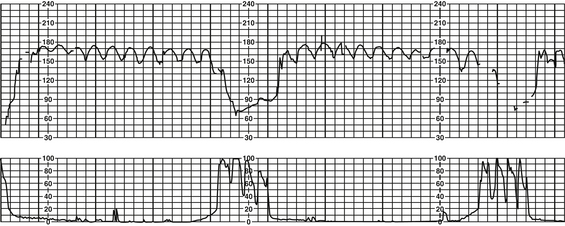
A Tachycardia (FHR >160 bpm for ≥10 minutes)
1. History: possible causes (Curran & Torgersen, 2006; Nageotte & Gilstrap, 2009; Simpson, 2008a; Tucker et al, 2009)
(6) Endogenous adrenaline or anxiety
(7) Medication or drug response
(a) Parasympathetics (Hydroxyzine [Visteril/Atarax], atropine, and phenothiazines)
(c) Beta-sympathomimetic drugs (Terbutaline; Ritodrine)
(d) Sympathomimetic bronchodilators used in asthma patients (albuterol)
(f) Selected positive inotropes (e.g., dobutamine, positive chronotropic drugs)
(g) Over-the-counter medications (e.g., decongestants, appetite suppressants, stimulants or caffeine)
(8) Illicit drugs (cocaine; methamphetamines)
(10) Nicotine, if inhaled (if inhaled by smoking, may increase the FHR; if absorbed through nicotine patch, may decrease FHR) (Lyndon et al, 2009; Muller, Antunes, Behle, Teixeira, & Zielinsky, 2002; Oncken et al, 1997; Oncken, Kranzler, O’Malley, Gendreau, & Campbell, 2002)
b. Fetal causes (Lyndon et al, 2009; Simpson, 2008a)
2. Physical findings (Figure 12-2)
a. Persistent FHR greater than 160 bpm for duration of a minimum of 10 minutes with no maximum (days or weeks are possible); if persists at greater than 200 to 220 bpm for extended period, can result in fetal hydrops or fetal demise (Curran & Torgersen, 2006; Lyndon et al, 2009).
b. Decreased variability common as rate increases, especially at rates greater than 180 bpm (Barnes, 2009; Torgersen, 2003)
c. Tachyarrhythmias (FHR >240 bpm, i.e., SVT) and ventricular arrhythmias more frequent
d. Tachycardia with moderate variability and in the absence of FHR decelerations rarely denotes fetal acidemia (Krebs, Petres, Dunn, Jordaan, & Segreti, 1979; Nageotte & Gilstrap, 2009).
b. Decreased parasympathetic tone
c. Persistently high rate associated with fetal hydrops or fetal demise (e.g., SVT, atrial flutter, atrial fibrillation)
a. Monitor maternal vital signs, specifically temperature and pulse.
b. Increase or initiate hydration with intravenous fluids as needed.
c. Initiate interventions to decrease maternal temperature, if elevated (e.g., antipyretics).
d. Assess for possible tachyarrhythmias or dysrhythmias; administer appropriate medications to lower FHR (e.g., cardiac agents for SVT, tocolytic agents or discontinuation of uterine-stimulating agents to enhance placental blood flow).
e. Perform scalp stimulation for tachyarrhythmias or dysrhythmias.
(1) Vagal stimulation may lower tachycardic FHR.
(2) FHR accelerations following scalp stimulation usually rule out an acidotic fetus.
(3) Fetal scalp stimulation is not appropriate in the presence of decelerations or bradycardia.
f. Reduce anxiety, offer explanations, provide comfort measures, and assist with breathing and relaxation techniques (Simpson, 2009).
g. Change or maintain maternal position that optimizes uteroplacental perfusion (e.g., side lying, walking).
h. Administer oxygen (10 to 12 L/min via rebreather face mask) as needed.
i. Assess for use of illicit and legal drugs and alcohol.
j. If auscultating, intervene as needed and consider application of EFM to further assess FHR, variability, and periodic and episodic changes.
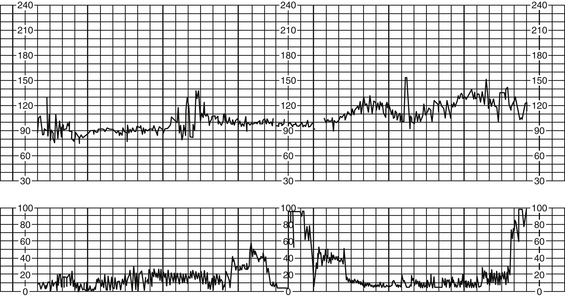
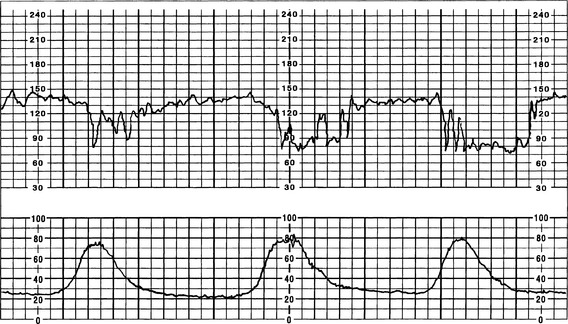
B Bradycardia (FHR <110 bpm for ≥10 minutes) (Figure 12-3) (Curran & Torgersen, 2006; Nageotte & Gilstrap, 2009; Simpson, 2008a; Tucker et al, 2009)
b. Anesthetic agents (e.g., epidural, spinal, pudendal, paracervical)
c. Adrenergic-receptor blocking agents (e.g., propranolol)
d. Connective tissue disease (e.g., systemic lupus erythematosus) (Hohn & Stanton, 2002; Tucker et al, 2009)
e. Prolonged maternal hypoglycemia
f. Conditions that cause acute maternal cardiopulmonary compromise (e.g., pulmonary embolus, anaphylactoid syndrome of pregnancy [formally amniotic fluid embolism], trauma, uterine rupture)
(1) Mature parasympathetic nervous system
(5) Congenital complete heart block (CCHB)
(6) Cardiac structural defect (resulting from maternal cytomegalovirus infection)
(8) Excessive parasympathetic nervous system tone (i.e., vagal stimulation) resulting from chronic head compression in a vertex presentation, occiput posterior, or transverse position; FHR usually does not decrease to less than 90 to 100 bpm in this situation (Lyndon et al, 2009; Simpson, 2008a)
a. FHR less than 110 bpm for duration of 10 minutes
b. Bradycardia accompanied by adequate variability may be normal (fetal dependent).
c. Prolonged deceleration resulting in change in FHR BL to bradycardia can be due to a sudden drop in oxygenation (as occurs with abruptio placentae), a decrease or occlusion in umbilical blood flow (cord prolapse or uterine rupture), or a decrease in uterine blood flow (significant maternal hypotension). This type of pattern can be indicative of fetal hypoxemia and may require immediate intervention (Nageotte & Gilstrap, 2009).
d. Moderate variability less likely if FHR persists at rate less than 90 bpm for more than 10 minutes; if variability and accelerations present, is considered benign or reassuring and not associated with acidemia (Freeman, Garite, & Nageotte, 2003; Simpson, 2008a).
e. Bradycardia accompanied by loss of variability and late decelerations may indicate current or impending fetal hypoxia (NICHD, 1997).
a. Excessive parasympathetic nervous system tone
b. Rate of approximately 60 to 70 bpm in second trimester and 50 to 60 bpm at term without variability may indicate CCHB (Eronen, Heikkila, & Teramo, 2001).
c. Bradycardia may be related to maternal hypotension secondary to supine hypotension, hypovolemia, vasodilation following epidural anesthesia, or maternal catecholamine production.
d. The lower the FHR, the lower the fetal cardiac output (Curran & Torgersen, 2006; Lyndon et al, 2009; Nageotte & Gilstrap, 2009).
e. Bradycardia less than 60 bpm or associated with decreased variability requires immediate attention and collaborative management (Curran & Torgersen, 2006; Lyndon et al, 2009; Nageotte & Gilstrap, 2009).
f. Rate must be differentiated from maternal rate (use real-time ultrasound or palpate maternal apical pulse and compare to FHR).
g. Rate must be differentiated from prolonged deceleration (prolonged deceleration duration is more than 2 minutes but less than 10 minutes; if deceleration is more than 10 minutes, bradycardia occurs).
a. Assess maternal vital signs, specifically BP.
b. Validate FHR versus maternal HR.
d. Perform vaginal examination for possible cord prolapse; if prolapse evident, elevate fetal presenting part off of cord.
e. note: Scalp stimulation has been noted as an intervention for bradycardia. In a nonreassuring FHR (bradycardia or prolonged decelerations without variability or accelerations), scalp stimulation is not recommended. Scalp stimulation in this instance will elicit a vagal response that overrides the sympathetic response and results in a further drop in the FHR (Curran & Torgersen, 2006; Harvey, 1987; Tucker et al, 2009).
Variability
Variability is defined as “the fluctuations in the FHR over time” and “is present when there are irregular fluctuations in the baseline FHR (Lyndon et al, 2009, p. 109).“These fluctuations are irregular in amplitude and frequency and are visually quantitated as the amplitude of the peak-to-trough in beats per minute” (Curran & Torgersen, 2006, p. 156). The two branches of the autonomic nervous system (parasympathetic and sympathetic) have opposite effects on the FHR. The parasympathetic nervous system slows the FHR, and the sympathetic nervous system speeds the FHR. This continual push-and-pull effect produces the moment-to-moment change in the FHR that is called variability. Variability is interpreted as a single combined term (NICHD, 1997); however, it is important to understand the different types of variability, specifically LTV and STV. These two types provide the visual tracing that is recorded on the EFM.
1. Described as normal irregularity of cardiac rhythm
2. Influenced by fetal oxygenation status, cardiac output regulation, fetal behavior during fetal sleep-wake states, humoral regulation, and drug effects (Freeman et al, 2003; Martin, 1982; Parer, 1997). Also influenced by alcohol and illicit drugs that can cause fetal neurologic damage thus affecting variability: morphine (Kopecky et al, 2000), methadone (Anyaegbunam, Tran, Jadali, Randolph, & Mikhail, 1997), anomalies, and previous insults damaging the fetal brain (Wadhwa, Sandman, & Garite, 2001)
3. Impulse transmission to the FHR is influenced by CNS oxygenation.
4. Adequate oxygenation and a mature and functioning autonomic nervous system contribute to production of variability (Lyndon et al, 2009).
5. Minimal variability may be associated with preterm fetus (less than 28 to 32 weeks’ gestation), alteration in the function of the nervous system, inadequate oxygenation, or any combination (Lyndon et al, 2009).
6. Minimal variability without associated decelerations is almost always unrelated to fetal acidemia (Parer & Livingston, 1990).
7. Moderate variability, even in the presence of decelerations, has a 98% association with an umbilical pH >7.15 or an Apgar score ≥7 at 5 minutes (Parer, King, Flanders, Fox, & Kilpatrick, 2006).
8. NICHD nomenclature describes and communicates variability as one unit (versus LTV and STV); however, it is still important to understand the physiology of LTV and STV.
a. FHR BL variability is determined in a 10-minute window, excluding accelerations and decelerations. Variability is described in four categories (Macones et al, 2008):
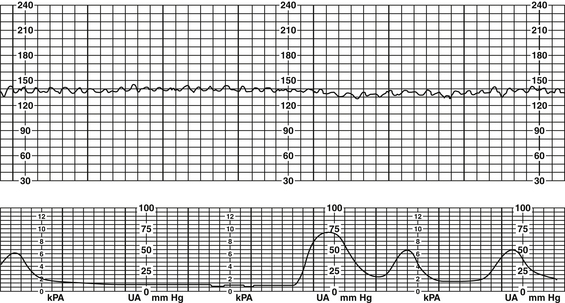
1. Defined as “having a visually apparent, smooth, sine wave-line undulating pattern in FHR baseline with a cycle frequency of three to five per minute that persist for ≥20 minutes” (Macones et al, 2008, p. 662)
2. In the presence of a true sinusoidal pattern the BL rate is usually within 110 to 160 bpm, frequency of two to five cycles, and amplitude undulations 5 to 15 bpm above and below the baseline (Simpson, 2008b).
3. A sinusoidal-like pattern can be seen following the administration of some drugs (i.e., Stadol, Fentanyl) and will resolve once the drug has been excreted (Curran & Torgersen, 2006; Simpson, 2008b). However, this pattern differs from the true sinusoidal pattern in that there are fewer and less uniform oscillations, periods of moderate variability and the presence of acceleration patterns (Curran & Torgersen, 2006; Simpson, 2008b) (Figure 12-7).
4. Sinusoidal characteristics (Figure 12-8)
a. Persistent oscillating pattern; BL rate 110 to 160 bpm
b. Amplitude of undulations usually 5 to 15 bpm greater than and less than BL rate
c. Frequency of undulation usually two to five cycles per minute
e. No accelerations present even in response to fetal stimulation or fetal movement
Fetal Heart Rate Patterns
Fetal heart rate patterns are described as periodic (associated with uterine contractions) or episodic (not associated with uterine contractions) (Macones et al, 2008). Periodic and episodic patterns can be distinguished by the waveform, whether abrupt or gradual, and are described by the NICHD as such. Additionally, the FHR patterns can also be described as recurrent, repetitive or intermittent (Curran & Torgersen, 2006; Macones et al, 2008). These terms are defined below:
1. Gradual: From the onset to the nadir (lowest part of the deceleration) is ≥30 seconds in duration.
2. Abrupt: From the onset to the peak or nadir is <30 seconds in duration.
3. Recurrent: If FHR patterns (periodic or episodic) occur with >50% of the uterine contractions in any 20-minute segment.
4. Repetitive: Periodic or episodic FHR patterns that occur with every uterine contraction (i.e., 100% of time) (Curran & Torgersen, 2006; NICHD, 1997).
5. Intermittent: Periodic or episodic FHR patterns that occur with <50% of the uterine contractions (Curran & Torgersen, 2006; Macones et al, 2008; NICHD, 1997).
PERIODIC PATTERNS
Periodic patterns are FHR patterns that have a direct relation to uterine contractions. Periodic patterns include accelerations and early, variable, and late decelerations.
Accelerations
1. Repetitive fetal stimulation or movement
2. Direct sympathetic stimulation
3. Mild umbilical cord compression stimulating fetal compensatory acceleration
B Physical findings (Figure 12-9)
1. Pattern characteristics (Curran & Torgersen, 2006; Macones et al, 2008; NICHD, 1997; Parer & Ikeda, 2007; Simpson, 2008b)
a. Term fetus: visually abrupt FHR increase defined as from the onset of acceleration to the peak of the acceleration in <30 seconds; the peak must be ≥15 beats above the BL FHR and must last a minimum of ≥15 seconds from onset to return
b. Preterm fetus <32 weeks’ gestation: visually abrupt (from onset to peak in <30 seconds) FHR increase from the onset of acceleration to the peak of the acceleration in <30 seconds; the peak acceleration defined as ≥10 beats above the BL FHR for a duration of ≥10 seconds
c. Prolonged acceleration: acceleration (as described above) lasting ≥2 minutes but <10 minutes
d. Variability is usually present.
e. Accelerations are considered to be benign patterns, indicate fetal well-being (well oxygenated), and require no interventions.
f. Shoulders: physiologic increase in the FHR before or after a variable deceleration secondary to an occlusion of the umbilical vein; increase in rate generally <20 bpm and lasting <20 seconds (Curran & Torgersen, 2006; Lyndon et al, 2009).
g. Overshoot: smooth, blunt, and prolonged acceleration following a late or variable deceleration; lasts more than 60 to 120 seconds with increase in rate 10 to 20 bpm; has no variability, no abruptness, and returns to BL FHR gradually; if repetitive with absent variability may require fetal intrauterine resuscitation (Curran & Torgersen, 2006; Lyndon et al, 2009)
a. When associated with fetal activity or stimuli that meet established criteria, accelerations are called reactivity.
(1) Observed in an active fetus or a fetus stimulated tactilely, by fetal scalp stimulation, or by vibroacoustic stimulation
(2) Fetal reactivity is expected by 28 to 30 weeks’ gestation; once fetus demonstrates reactivity, should continue through gestation.
b. When associated with variable decelerations, they are called shoulders or overshoots.
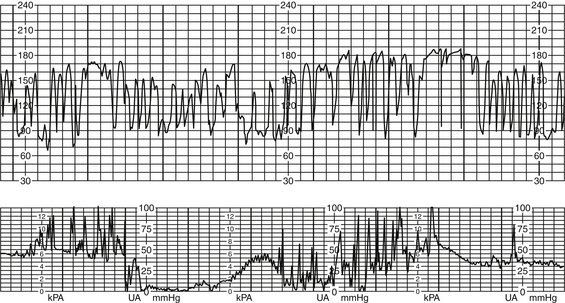
Variable Decelerations
1. Decreased umbilical cord perfusion
3. Baroreceptor stimulation with vagal response; depth of deceleration depends on fetal baroreceptor stimulation; depth is reflex mediated and not related to degree of fetal hypoxia or fetal acid-base status (Curran & Torgersen, 2006; Freeman et al, 2003; Lyndon et al, 2009)
4. Hypoxia and hypercarbic states
5. Associated with clinical observation of nuchal cord, body cord entanglement, prolapsed cord, short cord, decreased amniotic fluid, second-stage descent of fetus, true knot of cord, and decreased Wharton’s jelly
6. Acceleration that precedes or follows deceleration is a physiologic compensatory response to hypoxemia (oxygen deprivation).
1. Pattern characteristics (Curran & Torgersen, 2006; Macones et al, 2008; NICHD, 1997; Parer & Ikeda, 2007; Simpson, 2008b)
a. Visually apparent abrupt (from onset to beginning of nadir <30 seconds) decrease in the FHR below the baseline
b. Decrease in FHR is ≥15 bpm, lasting ≥15 seconds, and <2 minutes.
c. When periodic (associated with uterine contractions), onset, depth, and duration will vary with successive uterine contractions (Macones et al, 2008; NICHD, 1997) (Figures 12-10 and 12-11).
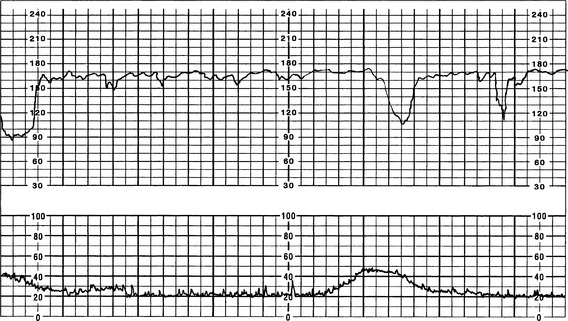
FIGURE 12-10 Periodic variable decelerations tolerated well by fetus as evidenced by recovery and moderate variability.
d. Shoulders and overshoots may be present with variable decelerations (see definition as previously noted).
1. Change maternal position to left or right lateral, upright, hands and knees, or knee-chest, whichever position relieves the FHR pattern.
a. Helps to relieve pressure on umbilical cord
b. Avoids supine hypotension and maximizes uteroplacental and umbilical blood flow
2. Administer oxygen if variable decelerations are recurrent, BL FHR is increasing or decreasing, and variability is absent, or if overshoots are present; oxygen administered via rebreather face mask at 10 to 12 L/min.
3. Perform vaginal examination to assess for prolapsed cord or imminent delivery; if prolapsed cord, elevate fetal presenting part off of umbilical cord.
4. Perform amnioinfusion according to institutional protocol.
a. Increases cushion effect for the umbilical cord
b. Relieves or lessens variable decelerations when effective
c. Dilutes thick, particulate meconium, if it is present
d. Sample protocol (also refer to own institutional guidelines)
(1) Most protocols recommend initial bolus of 800 mL; follow bolus with maintenance infusion to replace lost amniotic fluid (ACOG, 2005; Simpson, 2009).
(2) Some protocols recommend titration of fluid bolus at 15 to 20 mL/min until deceleration resolves, followed by an additional 250 mL.
(3) Warming of solution is not required for full-term fetuses; appropriate for preterm or growth-restricted fetuses; keep temperature between 34° and 37° C (93° and 96° F) (Simpson, 2009).
(4) Infusion may be discontinued when variables are abolished, the meconium is diluted, 800 mL is infused, or the amniotic fluid index (AFI) is used to determine infusion amounts.
(5) Maintenance fluid is 120 to 180 mL/hr (Simpson, 2009).
(6) Amnioinfusion should reach therapeutic result or increase the AFI in approximately 30 minutes (ACOG, 2005; Snell, 1993).
(7) It is important to monitor maternal vital signs, FHR and variability, resolution of decelerations, and uterine tone.
e. Discontinue oxytocin or other uterotonics; obtain order for and administer tocolytics (terbutaline) if UA continues.
g. Perform scalp stimulation if baseline is normal range and decelerations are not recurrent. Do not perform scalp stimulation during deceleration.
h. Instruct mother to alter her breathing or pushing technique.
i. Communicate findings to primary care provider, and document events on the patient’s record.