. Despite this, providing coordinated care by a knowledgeable interprofessional team of physicians, nurses, nurse practitioners, physician assistants, pharmacists, rehabilitation therapists, radiology technicians, dietitians, respiratory therapists, case managers, social workers, and others can make the difference in the functional outcome of the individual patient with ICH.
The intent of this chapter is to provide an overview of the incidence and prevalence of spontaneous ICH, differentiate commonly used terms associated with this disease, list primary and secondary causes, discuss early diagnosis and diagnostic tools, review the continuum of care, summarize available medical and surgical treatments, examine predictors for prognosis, and outline posthospital care coordination.  Unlike other chapters written on ICH, the goals are also to highlight implications for the interprofessional team, include practical tips for caring for this patient population, and provide tools that can be implemented in practice. The interprofessional team collaborates closely with each discipline, bringing their expertise to the group to provide comprehensive care to the patient with an ICH.
Unlike other chapters written on ICH, the goals are also to highlight implications for the interprofessional team, include practical tips for caring for this patient population, and provide tools that can be implemented in practice. The interprofessional team collaborates closely with each discipline, bringing their expertise to the group to provide comprehensive care to the patient with an ICH.
 BACKGROUND
BACKGROUND
Spontaneous nontraumatic ICH is a major public health problem that affects over 2 million people worldwide each year. Although there are variations related to geography and race, ICH is believed to account for 10% of all strokes each year (Go et al., 2013). Asians are two times more likely than other ethnic groups to suffer from an ICH. Patients of African, Hispanic, and Native American decent have higher rates of ICH than Caucasian Americans (Mohr et al., 2011). Medically indigent people without easy access to medical care and blood pressure control are at higher risk (Daroff, Fenichel, Jankovic, & Mazziotta, 2012). ICH admissions to the acute hospital have increased due to factors such as the advancing age of the U.S. population, uncontrolled hypertension, and the increased use of anticoagulants and antiplatelet agents (Qureshi, Mendelow, & Hanley, 2009).
 INTRACEREBRAL HEMORRHAGE QUALITY OF CARE
INTRACEREBRAL HEMORRHAGE QUALITY OF CARE
In 2002, about 77% of U.S. counties lacked hospitals with neurological services (Go et al., 2013). To address the need for a more organized approach, primary and comprehensive stroke centers have emerged. Primary stroke centers (PSCs) provide acute care to most stroke patients, whereas some patients need specialized care not available at PSCs. Comprehensive stroke centers (CSCs) are tasked with providing specialized care to the more complex patients. CSCs tend to have a higher percentage of hemorrhagic strokes because of transfers from outside facilities. A University of Texas study found that the majority of the patients transferred to the CSC were ICH cases (Albright et al., 2013). CSCs are required to have neurocritical care units, an intensivist available 24/7, protocols, policies, order sets, and acute stroke teams that respond promptly to manage acute stroke patients.
 TERMS AND DEFINITIONS
TERMS AND DEFINITIONS
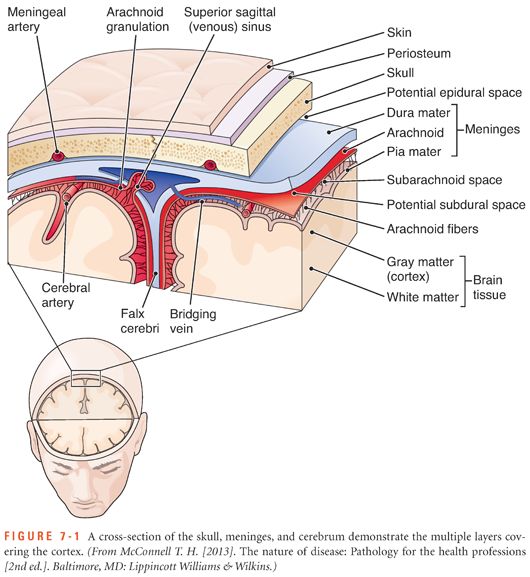
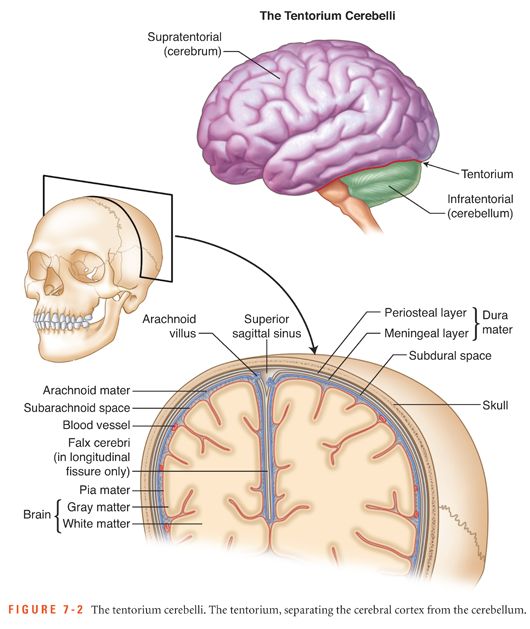
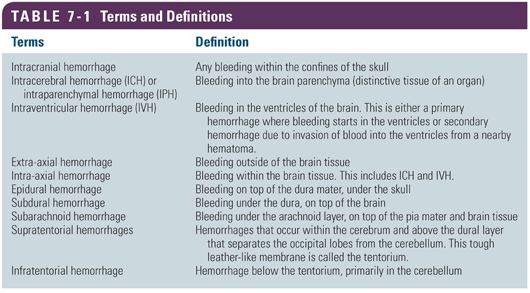
It is important to differentiate the types of hemorrhage within the cranium because each type has different causes and management (shown in Figs. 7-1 and 7-2 and listed in Table 7-1).
 CAUSES OF SPONTANEOUS NONTRAUMATIC INTRACEREBRAL HEMORRHAGE
CAUSES OF SPONTANEOUS NONTRAUMATIC INTRACEREBRAL HEMORRHAGE
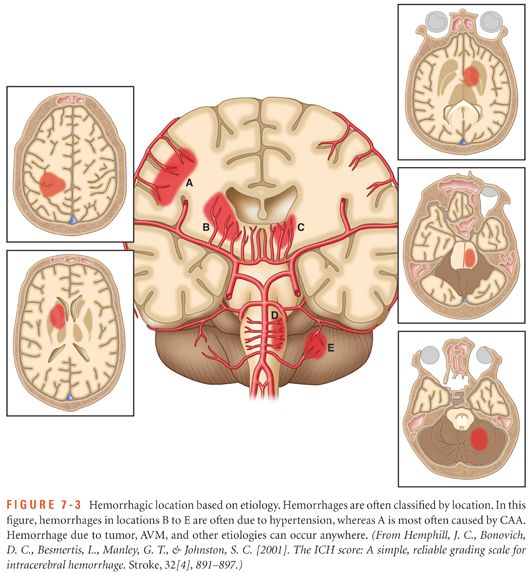
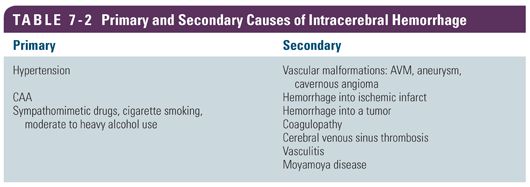
Depending on the underlying cause of the hemorrhage, ICH is classified as primary or secondary. Primary ICH originates from the spontaneous rupture of small arterioles damaged by chronic hypertension and cerebral amyloid angiopathy. Additionally, modifiable risk factors including diabetes mellitus, cigarette smoking, heavy alcohol use, and illicit drug use can also lead to blood vessel damage and rupture. Secondary ICH is associated with vascular malformations, abnormal coagulopathies, bleeding into a tumor or ischemic stroke, cerebral venous sinus thrombosis, vasculitis, or moyamoya disease. Figure 7-3 shows the typical hemorrhage location based on etiology and Table 7-2 provides a summary of primary and secondary causes of ICH.
Primary Causes
Hypertension
ICH is tragic and costly and, in many cases, preventable. Despite the availability of potent antihypertensive medications, many of which are on the “4-dollar list” at local pharmacies, hypertension is the leading cause of ICH (Qureshi & Palesch, 2011; Qureshi et al., 1997). Chronic hypertensive “wear and tear” of vessel degeneration at arterial bifurcations is by far the leading cause of primary ICH, especially in patients between the ages of 40 and 70 years. The vessel wall weakens and ruptures, and blood leaks into the surrounding tissue, causing a mass effect and toxic effects of blood products on the surrounding tissue. Elevated arterial pressure damages the intimal and subependymal layer of the arteriole, and the vessel becomes diseased and degenerates (Aguilar & Brott, 2011). Tiny end-vessel arteries, called penetrating arteries, which bifurcate from the larger more muscular vessels, are less equipped to handle the persistent abuse of high blood pressure. Charcot-Bouchard aneurysms (also known as microaneurysms) were described in neuropathological studies of brain specimens in 1868 in patients with ICH (Mohr et al., 2011). Hypertensive hemorrhages tend to result in a smaller volume of hematoma (≤30 cc) (Lang et al., 2001).
Common arterial locations for vascular disease are the lenticulostriate arteries and the pontine arteries. The lenticulostriate arteries come off the first branch of the middle cerebral artery and perfuse the deep structures of the brain including the basal ganglia, internal capsule, and thalamus. The lenticulostriate arteries are nicknamed “the arteries of stroke” because they can thrombose and cause an ischemic lacunar infarct or they may rupture under pressure and cause a classic deep hypertensive hemorrhage. The pontine perforators arise from the basilar artery and dive deep into the belly of the pons. Pontine hemorrhage is very often fatal.
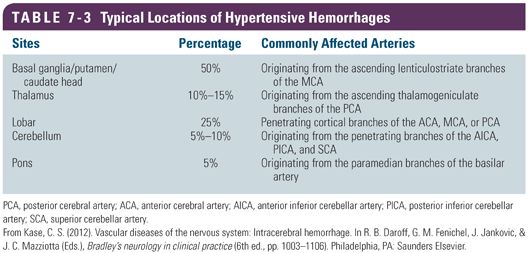
Deep hemorrhages in the basal ganglia, the internal capsule, and the pons account for about two thirds of spontaneous ICH cases and occur in the classic locations for hypertensive hemorrhages. Lobar hemorrhages at the junction of white and gray matter account for the remaining one third (Flaherty et al., 2005; Grysiewicz, Thomas, & Pandey, 2008). Although hypertension is exclusively associated with deep hemorrhages, there is evidence that lobar hemorrhages can also be attributed to hypertension (Broderick, Brott, Tomsick, & Leach, 1993). Table 7-3 lists a summary of typical locations of hypertensive hemorrhages.
Cerebral Amyloid Angiopathy
Cerebral amyloid angiopathy (CAA) is a disease process that primarily affects the elderly, and it is the second leading cause of ICH. In patients older than 65 years of age, CAA may exceed hypertension as the cause of ICH. CAA is caused by deposits of beta-amyloid peptides on the media and adventitial layers of the small- and medium-sized arteries in the brain and the leptomeninges (the pia and arachnoid layers of the meninges). The protein deposits adhere to the intimal wall of the blood vessel, causing degeneration of the vessel. In severe cases of CAA, the vessels may weaken to the point of rupture. Hemorrhages related to CAA tend to be larger, and the volume is often greater than 30 cc (Lang et al., 2001). These hemorrhages occur at irregular and unpredictable intervals, are predominantly lobar, and occur in different vascular territories. The gradient echo sequence of a magnetic resonance imaging (MRI) scan may show remnants of blood by-products of various ages in multiple vascular distributions. This picture is highly suggestive of CAA. However, CAA is a diagnosis of exclusion and can only be confirmed with a brain biopsy or during autopsy.
CAA is also thought to be associated with the genetic risk factor apolipoprotein E (ApoE). Other risk factors include leukoaraiosis (white matter disease), a family history of ICH, a previous ischemic stroke, and frequent alcohol use. CAA also has similar histopathological features of Alzheimer disease (Mohr et al., 2011). White matter disease is commonly seen in elderly patients and in patients with hypertension, and a correlation was found between white matter disease and amyloid-positive vessels (Gatchev et al., 1993). White matter disease is also associated with larger hematoma volume and the risk of expansion (Lou, Al-Hazzani, Goddeau, Novak, & Selim, 2010). A familial association has been recognized in young patients and is thought to be related to a mutant protein deposit that is unrelated to beta-amyloid but which promotes vascular dysfunction in a similar fashion (Levy, Lopez-Otin, Ghiso, Geltner, & Frangione, 1989).
Other Primary Causes
In addition to hypertension and CAA, other diseases such as diabetes mellitus and personal habits such as cigarette smoking affect the cerebral vasculature. Patients with diabetes are at a three- to fourfold increased risk for ICH due to macrovascular and microvascular complications of the disease (Kissela et al., 2005). Cigarette smoking is a well-established risk factor for both ischemic and hemorrhagic stroke. Heavy alcohol use, another risk factor, is thought to inhibit platelet function, disturb coagulation pathways, and raise blood pressure in a linear dose-response fashion (Juvela, Hillbom, & Palomäki, 1995). Illicit drug use, with stimulants such as methamphetamines and cocaine, are associated with ICH. The cause may be abrupt spikes in blood pressure and cardiac arrhythmias. Interestingly, former cocaine users are more likely to have large vessel atherosclerosis, but current illicit drug users are more likely to suffer from an ICH stroke (Toossi, Hess, Hills, & Josephson, 2010). The positive relationship to hypercholesterolemia is less clear in ICH than in ischemic stroke. Contrary to ischemic stroke risk, high cholesterol actually seems to decrease the risk of ICH. This may indicate that atherosclerosis is not the predominant cause of ICH; rather, fragility of the vessels is the underlying reason for the rupture of the vessel (Ariesen, Claus, Rinkel, & Algra, 2003).
Secondary Causes
Vascular Anomalies
Rupture of vascular anomalies such as arteriovenous malformations (AVMs), cerebral aneurysms, or cavernous angiomas can lead to ICH. Hemorrhages of vascular anomalies occur more frequently in the younger, predominately female population and tend to follow a distinctive pattern of blood product markings on scans. Other distinctive characteristics include associated subarachnoid hemorrhage or intraventricular hemorrhage (IVH), prominent vascular structures, calcifications, and atypical locations for hypertensive ICH (Daroff et al., 2012). Cerebral angiography and/or MRI/magnetic resonance angiography (MRA) should be performed to definitively identify an underlying lesion.
Oral Anticoagulation
Cerebral hemorrhage is a major concern for patients receiving oral anticoagulation therapy (OAT). There are multiple validated indications for prescribing OAT; the number of patients receiving full-dose anticoagulation has increased. Fully anticoagulated patients have an 8- to 11-fold increase in the incidence of ICH, with the occurrence of death or severe disability about 65% (Daroff et al., 2012). Combining anticoagulants such as warfarin or the new oral anticoagulants such as dabigatran, rivaroxaban, or apixaban with aspirin increases the risk of ICH twofold.
Mortality is substantially higher in warfarin-induced ICH in an associated dose-dependent correlation. Careful control of the international normalized ratio (INR) can limit the risk and the severity of ICH. The onset of stroke-like symptoms in elderly patients receiving OAT warrants urgent evaluation (Rosand, Eckman, Knudsen, Singer, & Greenberg, 2004). The risk of ischemic and ICH strokes should be weighed prior to the initiation of full anticoagulation therapy. Patient and family education regarding dietary restrictions, underscoring the importance of INR monitoring, and recognizing signs and symptoms of ICH is critical. Provider documentation should clearly reflect initial education and ongoing reinforcement for all patients. A clear plan for follow-up laboratory surveillance and provider oversight should be arranged prior to discharge.
Hemorrhage into a Tumor
Intracranial tumors can be highly vascular and can lead to hemorrhage. The most common bleeding metastatic intracranial tumor is a metastatic melanoma. MRI scans are helpful when trying to identify a lesion under the hematoma (Daroff et al., 2012). For patients with either papilledema noted at the presentation of an acute ICH, an atypical location of ICH, or a ring-like high-density hemorrhage enhancement on the scant, an underlying tumor should be suspected.
Hemorrhage into an Ischemic Infarct
Hemorrhagic transformation of an ischemic stroke can occur spontaneously or after administration of tissue plasminogen activator (tPA) for acute ischemic stroke symptoms. Approximately 10% of patients with an ischemic stroke will have some hemorrhage within the stroke bed. The hemorrhage can range from a petechial hemorrhage to a parenchymal space-occupying hemorrhage with a large volume of blood. Prior to giving intravenous (IV) tPA, the risk for intracranial hemorrhage should be discussed with the patient and family. For patients who present within 3 hours of symptom onset, approximately 6% will have a symptomatic ICH compared to a rate of 0.6% of symptomatic ICH in the control group. These hemorrhages are classified as either symptomatic or asymptomatic based on clinical deterioration (Paciaroni, Bogousslavsky, & Levine, 2013). A symptomatic hemorrhage is defined as intracerebral blood along with a 4-point worsening in the National Institutes of Health Stroke Scale (NIHSS). Symptomatic ICHs are more commonly associated with higher NIHSS scores, cerebral edema, or mass effect on the baseline head computed tomography (CT) scan. An NIHSS score higher than 20 has a 17% change of hemorrhage (The NINDS t-PA Stroke Study Group, 1997). The European Cooperative Acute Stroke Study (ECASS III) clinical trial conducted in Europe showed that IV tPA was safe to be given up to 4.5 hours from onset of symptoms (Hacke et al., 2008). The risk of symptomatic hemorrhage in this expanded time window is 8%. Hospitals of every size and location can safely treat stroke victims with IV tPA if they have access to consultation and transfer agreements with experienced stroke centers.
Other Secondary Causes
Other less common secondary causes of ICH include a venous hemorrhage related to cerebral venous sinus thrombosis (CVST), vasculitis, and moyamoya disease. ICH occurs in the setting of CVST due to the blockage of the normal outflow of the venous blood via the venous sinuses. This buildup of pressure in the venous system can lead to rupture of the nearby veins and formation of a hematoma. The treatment for CVST is full anticoagulation, which is difficult when there is intracranial bleeding already present.
Another secondary cause, vasculitis, is characterized by inflammation and necrosis of the cerebral vessels and is mainly associated with autoimmune disorders such as giant cell arteritis and classic polyarteritis nodosa. These inflamed and necrotic vessels become damaged and can hemorrhage. The laboratory workup for inflammation includes an increased sedimentation rate and C-reactive protein (CRP) levels. Conventional angiography may show signs of large vessel angiitis. MRI, including apparent diffusion coefficient (ADC) maps, diffusion/perfusion measurements, and gradient echo sequence, can show evidence of both ischemic and hemorrhagic lesions of varying ages. Lumbar puncture and analysis of cerebrospinal fluid (CSF) may be performed if vasculitis is suspected and if the laboratory findings are inconclusive (Berlit, 2010).
Moyamoya disease is a chronic and progressive vasculopathy that presents with intracranial hemorrhagic or ischemic stroke. The term moyamoya, the Japanese word for “puff of smoke,” describes the angiographic appearance of fragile collateral vessels that develop adjacent to severely stenotic cerebral arteries. The intima has duplicate or even triplicate layers of elastic lamina, which may be related to proliferation of smooth muscle cells in the vessel. These thin and dilated collaterals may develop vessel wall necrosis and microaneurysms and are the likely etiology of hemorrhages. Morbidity and mortality for patients with moyamoya disease with ICH is substantial (Zipfel, Fox, & Rivet, 2005).
 DIAGNOSIS WITH COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
DIAGNOSIS WITH COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
Imaging with CT or MRI is the only definitive way to differentiate ischemic stroke from hemorrhagic stroke because symptoms can be similar. A CT scan without contrast medium is the imaging modality of choice in the diagnosis of acute ICH. CT scans without contrast media are highly sensitive and specific for the detection of acute blood products in the brain and can typically be done very quickly. CT is less sensitive in detecting subacute and old ICHs (Mohr et al., 2011). Most hospitals, even critical access hospitals with less than 25 beds, will have a CT scanner readily available 24/7. When viewing a noncontrast CT, the hyperdense appearance of fresh blood is easy to detect. If available, obtaining a CT angiogram (CTA) is helpful for detecting an underlying aneurysm, AVM, or venous thrombosis. Cerebral angiography should be obtained if clinical suspicion of a vascular anomaly is high or if there is an unusual appearance of the hemorrhage (Morgenstern et al., 2010). In addition, a CTA can help predict hematoma growth early in the course of illness. By using the CTA source images, a “spot sign” or contrast leaking in the vessel that appears hyperdense compared to the surrounding hematoma may be detected (Mohr et al., 2011).
The MRI is also recognized for its accuracy in detection of blood products as well as identification of underlying lesions such as intracranial tumors, cerebral vein thrombosis, cavernous angiomas, and other vascular malformations. The appearance of blood on an MRI depends on the evolution of the blood breakdown products. Several studies have demonstrated that an MRI is as reliable as noncontrast CT in the detection of acute blood. However, patients must be able to tolerate lying still for a longer period of time and screened for any contraindications for the MRI. In patients without a clear underlying etiology or in hemorrhages occurring in unusual locations, an MRI with contrast is indicated.
Clinical Pearl: Radiology
● An MRI checklist must be completed to check for any contraindications for the study. Obtain information about contraindications for an MRI from the family, when they are available, in order to avoid having to track them down if an MRI is ordered.
● Safely transporting critically ill patients requires coordination by multiple team members. The central coordinator of this activity is the primary nurse who will need to alert the charge nurse, call the radiology technicians, arrange transportation help, and notify respiratory therapy who will need to bring a portable ventilator for transport.
● Ordering serial CT scans in the first 24 hours is a common practice. Anticipate that the patient will need to be transported several times during the first few days of hospitalization.
● “Three B’s”—Blood, bone, and bling appear white on CT images.
 THE CONTINUUM OF INTRACEREBRAL HEMORRHAGE STROKE CARE
THE CONTINUUM OF INTRACEREBRAL HEMORRHAGE STROKE CARE
Stroke Prevention: Beginning the Continuum
Hypertension is one of the most preventable contributors of ICH. Rigorous review of randomized controlled trials is summarized, and recommendations are provided in the Eighth Joint National Committee (James et al., 2014) on the management of hypertension.  These recommendations are designed to provide clear guidance especially for the primary care clinician who is the gatekeeper for treatment of hypertension. The JNC 8 recommendations include a stepwise progression of drug classes based on individual demographics and response to treatment (James et al., 2014). Close monitoring of anticoagulant doses with appropriate titration by a skilled provider also reduce the risk of ICH. Anticoagulation clinics have been shown to reduce the number of bleeding events and the number of thromboembolic events as well as decrease cost by reducing the number of hospitalizations and emergency department visits (Chiquette, Amato, & Bussey, 1998). Patients with known CAA should not be placed on any antithrombotic therapy if possible, and caution should be used in initiating antithrombotic therapy in patients with large ischemic strokes. Lifestyle modifications including avoidance of sympathomimetic drugs and cigarette smoking as well as controlling blood glucose can decrease the chance of an ICH.
These recommendations are designed to provide clear guidance especially for the primary care clinician who is the gatekeeper for treatment of hypertension. The JNC 8 recommendations include a stepwise progression of drug classes based on individual demographics and response to treatment (James et al., 2014). Close monitoring of anticoagulant doses with appropriate titration by a skilled provider also reduce the risk of ICH. Anticoagulation clinics have been shown to reduce the number of bleeding events and the number of thromboembolic events as well as decrease cost by reducing the number of hospitalizations and emergency department visits (Chiquette, Amato, & Bussey, 1998). Patients with known CAA should not be placed on any antithrombotic therapy if possible, and caution should be used in initiating antithrombotic therapy in patients with large ischemic strokes. Lifestyle modifications including avoidance of sympathomimetic drugs and cigarette smoking as well as controlling blood glucose can decrease the chance of an ICH.
The Stroke Care Continuum: Prehospital to the Emergency Department
Early identification of stroke symptoms and mobilization of emergency medical services (EMS) are critical in order to avoid delays in care. Large public campaigns (e.g., the 5 suddens, F-A-S-T) were launched in order to increase public knowledge about stroke symptoms and to call 911 for urgent transport. Dispatch of EMS and rapid transportation to the closest appropriate hospital should be tracked as performance measures. Prehospital stroke assessment scales such as the Cincinnati Prehospital Stroke Scale and the Los Angeles Prehospital Stroke Scale are widely used to educate EMS providers. See Chapter 4 for further discussion of prehospital care.
Hippocrates, the father of medicine, first recognized the symptoms of stroke and called it apoplexy, which is translated as “struck down by violence.” The onset of focal neurological deficits with clinical signs of increased intracranial pressure (ICP) including severe headache, vomiting, loss of consciousness/coma, and systolic blood pressure greater than 220 mm Hg are strongly suggestive of ICH (Aguilar & Brott, 2011). Unlike ischemic stroke or subarachnoid hemorrhage, neurological symptoms related to ICH may not be at their maximum at onset. The hematoma can grow over minutes to hours, and symptoms progress and worsen depending on a mass effect on surrounding structures as different vascular territories become affected. Early hematoma growth and subsequent perihematomal edema contribute to increased ICP and deterioration in the neurological examination. Patients with hemorrhage below the tentorium may have signs of brainstem dysfunction, gaze abnormalities, cranial nerve deficits, and contralateral motor deficits. Ataxia, nystagmus, and dysmetria are common with cerebellar hemorrhages (Qureshi et al., 2001).
 All members of the interprofessional team should be diligent during this time to conduct frequent neurological assessments and monitor vital signs closely to assess for signs of increasing ICP due to hematoma expansion, mass effect, and/or development of hydrocephalus. Understanding the typical timeline for the development of complications will help the whole team in planning the course of treatment. Realizing that hydrocephalus and hematoma expansion frequently occurs in the first hours, but that cerebral edema does not peak until around day 5, helps the team members to focus on the probable causes of neurological changes.
All members of the interprofessional team should be diligent during this time to conduct frequent neurological assessments and monitor vital signs closely to assess for signs of increasing ICP due to hematoma expansion, mass effect, and/or development of hydrocephalus. Understanding the typical timeline for the development of complications will help the whole team in planning the course of treatment. Realizing that hydrocephalus and hematoma expansion frequently occurs in the first hours, but that cerebral edema does not peak until around day 5, helps the team members to focus on the probable causes of neurological changes.
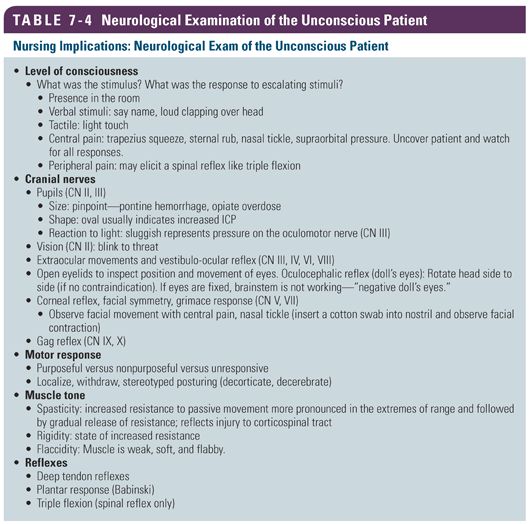
 Nurses are present at the bedside 24/7 during the hospitalization of a patient. One topic of debate is the neurological assessment tool and the frequency of the examination. Nurses should conduct a detailed neurological examination at the beginning of their shift and during handoff to an oncoming nurse. Best practice is to conduct a bedside assessment with both nurses present. Once the baseline examination is established and the deficits are identified, conducting a focused examination throughout the shift allows the nurse to quickly identify deterioration in the exam. Changes in the focused bedside examination should lead to repeating the detailed neurological examination (Table 7-4). Knowing what is normal and anticipating deficits based on knowledge of the location of the ICH will help the nurse complete both the focused and the detailed neurological examination. Although the NIHSS is most commonly used in the ischemic stroke population, it is also used to assess patients with intracranial hemorrhage (Hinkle, 2014).
Nurses are present at the bedside 24/7 during the hospitalization of a patient. One topic of debate is the neurological assessment tool and the frequency of the examination. Nurses should conduct a detailed neurological examination at the beginning of their shift and during handoff to an oncoming nurse. Best practice is to conduct a bedside assessment with both nurses present. Once the baseline examination is established and the deficits are identified, conducting a focused examination throughout the shift allows the nurse to quickly identify deterioration in the exam. Changes in the focused bedside examination should lead to repeating the detailed neurological examination (Table 7-4). Knowing what is normal and anticipating deficits based on knowledge of the location of the ICH will help the nurse complete both the focused and the detailed neurological examination. Although the NIHSS is most commonly used in the ischemic stroke population, it is also used to assess patients with intracranial hemorrhage (Hinkle, 2014).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


