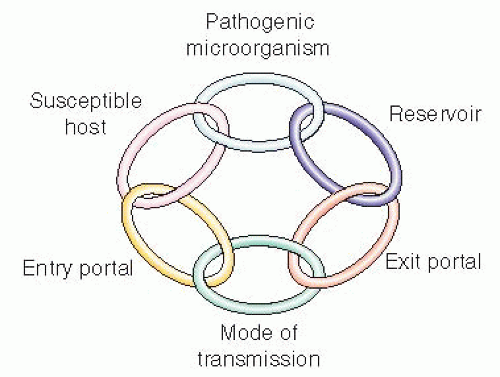
Pathogenicity—ability to produce disease.
Virulence—disease severity.
Infectious dose—number of organisms needed to initiate infection.
Toxigenicity—capacity to produce injurious substances that damage the host.
Adaptability—ability to adjust to changing conditions, ie, resistance to antimicrobial agents (see Table 31-1).
Human—pulmonary tuberculosis.
Animal—ticks infected with the bacteria Borrelia burgdorferi, which causes Lyme disease.
Environment—Legionnaire’s disease (Legionella pneumonphila) through water.
Fomite— Methicillin (oxacillin)-resistant Staphylococcus aureus bacteria on a bedside table.
Respiratory tract.
GI/GU tract.
Body fluids (except for sweat) such as blood, urine, semen.
Skin, mucous membranes.
Transplacental.
Table 31-1 Multidrug-Resistant Organisms (MDROs) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Direct contact—infected by touching the reservoir (eg, Clostridium difficile fecal-to-oral transmission or rubella virus crossing the placenta to the fetus).
Droplet transmission.
Droplets of large particles more than 5 microns in size usually from respiratory secretions.
Transmitted through coughing, sneezing, or talking to an infected person (eg, human metapneumovirus spread through coughing).
Transmitted through aerosolizing procedures (eg, sprays of infectious agents during a nebulization treatment or a sputum induction).
Airborne transmission.
Droplet nuclei—less than 5 microns that remains suspended in air (eg, tuberculosis and varicella [primary chickenpox]).
Dust particles in the air containing the infectious agent (eg, Aspergillus fungi through dust).
Common vehicle route (through contaminated items).
Food (eg, Salmonella and Campylobacter).
Water (eg, cholera and Legionellosis).
Medications (eg, hepatitis C infection from contaminated multidose vials).
Vectorborne transmission.
A living creature acts as an intermediary acquiring a pathogen from one living host and transmits the disease agent to another living organism, often an arthropod (fly, mosquito, tick).
Can be mechanical (carried on the surface) or biological (host is infected with pathogen) vectors.
Determined by a complex interrelationship between a host and an infectious agent, by factors that influence infection or disease, such as:
Pathogenicity—the ability to produce disease in a host. The organism invades a host, enters tissue, colonizes then spreads from host to host while not necessarily causing death to the host;
Virulence—the wide range of damage that can occur to the host because of the toxic capabilities of the pathogen. Given that the host-pathogen relationship is fluid, the outcome can be dictated by:
Number of pathogens to which the host is exposed; route and duration of exposure.
Invasiveness of the pathogen and its ability to produce toxins.
Age, genetic constitution of host, and general physical, mental, and emotional health and nutritional status of host.
Ability to bypass or overcome host defense mechanisms (immunologic response).
 Evidence Base
Evidence Base
It is imperative that specimens be collected and handled carefully if the causative agent for infection is to be identified correctly.
Specimens should be collected during the acute phase of infection and before the initiation of antibiotic therapy, if possible.
Obtain an adequate amount of the specimen necessary for all tests.
Avoid potential contamination of the specimen by using proper collection technique.
Check local laboratory guidelines for the specimen collection recommendations for each test. These guidelines should include the appropriate specimen containers, sample size, as well as transport requirements (temperature, time, etc.).
Label the container properly according to local laboratory protocol; in general, include the patient’s name, date of birth, medical record number, the source of the specimen, date and time collected, test to be performed, and any special instructions the provider may request.
Transport the specimen to the laboratory as soon as possible according to laboratory guidelines.
Be familiar with hospital policy recommending the transport of specified pathogens by staff personnel to the laboratory instead of via a pneumatic tube system.
Employers are required to provide appropriate personal protective equipment (PPE) and other engineering/work practice controls to eliminate or minimize employee exposure to bloodborne pathogens as mandated in OSHA regulation 1910.
Gloves are to be worn when there is anticipation of contact with blood, other potentially infectious materials, mucous membranes, and nonintact skin.
Gowns, aprons, or other protective body clothing are to be worn when contamination of clothing with blood or other potentially infectious materials is anticipated.
Masks in combination with protective eyewear are to be worn when performing procedures likely to generate sprays or splashes of blood or other potentially infectious materials into the eyes, nose, or mouth.
Hands and other skin surfaces are to be washed immediately if contaminated with blood or other potentially infectious materials.
Utilize safety devices for needles and other sharps and do not manipulate by hand (eg, recapping, purposely bending or breaking, or removing from syringes, etc.).
Needles and other sharps are to be placed in puncture-resistant containers for disposal.
All specimens and items with blood or other potentially infectious materials are to be transported in containers that prevent leakage.
Blood and body fluid spills are to be cleaned up promptly with an appropriate germicide, such as a bleach solution or phenolic.
Normally a sterile body fluid.
Specimens obtained by peripheral venipuncture are preferred over sampling from vascular catheters due to contamination of the catheter. Culturing hardware to determine a central line infection is not recommended in the literature.
Aseptic technique is essential to avoid contaminating the specimen with organisms colonizing the skin or the collector’s hands.
Cleanse the venipuncture site with 70% alcohol followed by chlorhexidine gluconate and allow to fully dry.
The diaphragm tops of the culture bottles are not sterile and must be cleaned with alcohol before injection of blood.
Normally a sterile body fluid.
A clean-catch, midstream urine collection provides the best method for obtaining a specimen to detect a urinary tract infection (see page 774).
Patients who are catheterized should have the specimen withdrawn using a sterile syringe from the catheter sampling port (see page 781).
Urine specimens must be transported to the laboratory promptly. If not cultured within 30 minutes of collection, urine must be refrigerated and cultured within 24 hours.
Obtained to culture organisms that are not part of the normal bowel flora (eg, Salmonella species, Shigella species).
Patient should defecate into a sterilized container or bedpan. Stool specimens should not contain urine or water from the toilet bowl.
Stool specimens can also be obtained directly from the rectum using a sterile swab.
Specimen needs to be from the lower respiratory tract, not oropharyngeal secretions. The laboratory will perform a Gram stain on all sputum specimens to determine if they are representative of pulmonary secretions and appropriately collected. A specimen containing a majority of cells from squamous epithelium may be rejected.
The most common method of collection is expectoration from a cooperative patient with a productive cough. Early morning is the optimal time to collect sputum specimens.
A sputum specimen can be collected in a sputum trap from patients who have artificial airways and require suctioning.
If a patient cannot produce sputum, sputum induction using an aerosol nebulizer may assist with loosening thickened secretions.
Bronchoscopy may be required to obtain sputum if induction fails.
Specimens are usually cultured for aerobic and anaerobic organisms.
Specimens may be collected by multiple techniques, depending on the depth of the wound, including tissue samples, needle aspiration, and swabbing the surface.
To collect a wound culture using a sterile swab, cleanse the surface and collect as much exudate as possible from the advancing margin of the lesion. Avoid swabbing surrounding skin.
Place the swab immediately in appropriate transport culture tube and send to the laboratory.
Label with the specific anatomic site.
Use a tongue depressor to hold the tongue down.
Carefully yet firmly rub swab over areas of exudate or over the tonsils and posterior pharynx, avoiding the cheeks, teeth, and gums.
Insert swab into packet and follow directions for handling the transport medium.
Microscopic examination distinguishes tissue cells from microorganisms.
Various stains are used to highlight the structural characteristics of microorganisms (eg, Gram stain, acid-fast stains to isolate mycobacteria).
Classification is conducted according to physical appearance such as shape, size, or tendency to form chains or clusters and stain reactions such as Gram-positive versus Gram-negative.
Results of microscopy are usually available within minutes, which permits early initiation of treatment based on a presumptive diagnosis.
Culture is the gold standard for positive identification of most microorganisms.
Different culture media are used for suspected pathogens and can be selective (allow growth of only certain microorganisms) and/or differential (distinguish between different bacteria based on different characteristics).
A liquid medium is used for blood specimens because lower numbers of microorganisms are detectable.
A solid medium is used to isolate mixtures of organisms and grow pure cultures of each type of organism found.
Recovery of pathogens from culture varies depending on the microorganism type, test selected, and stage of illness.
Most common pathogens, such as staphylococci, streptococci, and enterococci, are often identifiable to genus and species within 48 hours.
Fungal organisms may take 10 to 14 days to grow in culture medium.
Viruses may take 2 to 3 weeks to grow in culture medium.
Mycobacteria may take up to 6 weeks to grow in culture medium.
Used to determine minimal concentration of antibiotics that will inhibit growth of an organism.
Minimum inhibitory concentration is the lowest concentration of an antimicrobial drug that will inhibit organism growth and is measured by the size of the zone around the antimicrobial disk in which growth is inhibited.
Based on Clinical and Laboratory Standards Institute guidelines for each organism, the size of the inhibitory zone diffusion tests are interpreted as resistant (R), sensitive (S), and intermediate (I).
There are technologies to detect specific genetic portions of pathogenic organisms or to identify the specific host’s response to the presence of the pathogen. Examples include DNA probe testing and polymerase chain reaction.
These tests often yield a more rapid result than culture.
An increase in white blood cell count or “leukocytosis” may indicate infection, inflammatory response, tissue necrosis, or bone marrow failure.
The total number of circulating leukocytes and the differential (given as a percent of the total white blood cell [WBC] count) may change during a bacterial or viral infection.
During an acute bacterial infection, the WBC count often increases (greater than 11,000/mm3), accompanied by increased neutrophils and increased bands (immature neutrophils) in the differential. The shift (to the left) in differential reflects phagocytic activity.
Pathogens that are antigenic stimulate antibodies that can be detected in the serum of patients.
Detection of antibodies is not necessarily diagnostic of current infection.
Antigen-antibody reactions must be evaluated over a period of time.
Immunoglobulin M (IgM) antibody production peaks during active infection and decreases during convalescence.
IgG antibodies peak during convalescence and persist.
A fourfold rise in antibody titer between the acute and convalescence samples indicates recent infection.
Substance Isolation into a single set of precautions, called standard precautions. Standard precautions apply to (1) blood; (2) all body fluids, secretions, and excretions (except sweat), regardless of whether or not they contain visible blood; (3) nonintact skin; and (4) mucous membranes. As a common practice, standard precautions are recommended for the care of all patients in any environment, every time. If there is a chance of coming into contact with a potentially infectious material, a barrier is recommended to be placed between the patient and the care provider. The barrier may be a gown, glove, mask, or goggles, depending on the reason and area of contact.
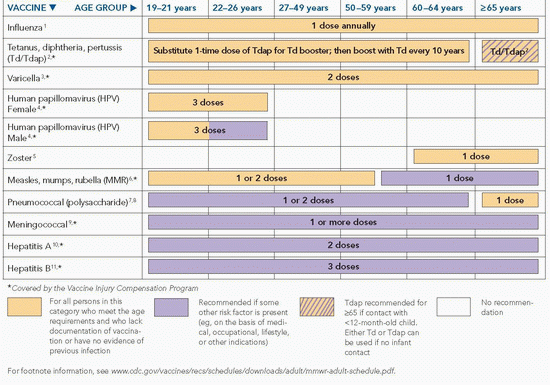
Table 31-2 Recommended Adult Immunization Schedule—United States, 2012 | |
|---|---|
|
Hand hygiene is the single most recommended measure to reduce the risks of transmitting microorganisms.
Hand hygiene should be performed between patient contacts; after contact with blood, body fluids, secretions, excretions, and contaminated equipment or articles; before donning and after removing gloves is vital for infection control. It may be necessary to clean hands between tasks on the same patient to prevent cross-contamination of different body sites.
To perform hand hygiene, clean hands with soap and water, applying friction for 15 seconds upon all surfaces of the hands, or applying alcohol-based waterless hand sanitizer covering all surfaces of both hands until completely dry.
Waterless hand cleaners are recommended unless there is visible soil on the hands, before eating, after using the restroom, and when there is significant buildup of waterless hand cleaners.
If caring for a patient with a spore-producing pathogen such as Clostridium difficile-associated disease (CDAD), then use hand hygiene with soap and water applying friction for 15 seconds, as the spores this organism forms are resistant to alcohol hand gel.
Similarly, for other pathogens known or suspected to be resistant to alcohol waterless hand gels—such as Norovirus— hand hygiene with soap and water while applying friction for 15 seconds.
 NURSING ALERT
NURSING ALERT
Used for patients known or suspected to be infected or colonized with epidemiologically significant microorganisms that can be transmitted by direct contact with the patient (skin-to-skin or patient’s skin to staff’s clothing as contact occurs when performing patient care activities that require touching the patient’s skin) or indirect contact with environmental surfaces (fomites) or patient care items in the patient’s environment.
Examples of microorganisms requiring contact precautions:
Methicillin (oxacillin)-resistant Staphylococcus aureus (MRSA).
Vancomycin-resistant Enterococcus.
Vancomycin-intermediate or -resistant S. aureus.
CDAD.
Place the patient in a private room or in a room with a patient who has the same microorganism (cohorting).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree