Are impermeable to bacteria and other contaminants
Facilitate autolytic debridement
Are self-adherent and mold well
Provide light to moderate absorption
Minimize skin trauma and disruption of healing
Allow observation of the healing process, if transparent
May be used under compression products (compression stockings, wraps, pumps, and Unna’s boot)
Are not recommended for wounds with heavy exudate, sinus tracts, or infections; wounds surrounded by fragile skin; or wounds with exposed tendon or bone
Can make wound assessment difficult, if opaque
May be dislodged if the wound produces heavy exudate
Provide an occlusive property that limits gas exchange between the wound and the environment
May curl at edges
May injure fragile skin upon removal
Contraindicated for patients with sensitivity to the dressing or its components
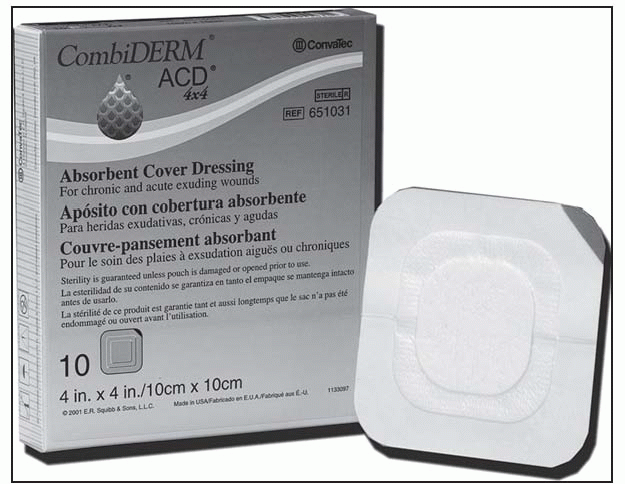
Clean the wound surface and surrounding skin with an appropriate cleansing solution. Dry the surrounding skin thoroughly.
Debride the wound, if necessary.
Determine the ideal dressing size, allowing a minimum 1¼″ (3 cm) margin beyond the reddened skin.
If a filler or exudate management product is required, apply AQUACEL Hydrofiber Dressing, KALTOSTAT alginate dressing, or other appropriate dressing.
Remove CombiDERM ACD release paper, and place the dressing directly over the wound.
Press and smooth the dressing edges to ensure adherence and a firm seal.
Gently press down on the skin, and carefully lift the blue tab on the corner of the dressing. Continue until all edges are free.
Carefully lift away the dressing.
Contraindicated for patients with sensitivity to the dressing or its components
Clean the wound site, rinse well, and dry the surrounding skin.
Choose a dressing that extends 1″ (2.5 cm) beyond the wound.
Apply the dressing, white side down, directly over the wound.
Secure the dressing with tape or with a secondary bandage or wrap.
For highly exuding wounds, it’s recommended that CombiDERM Non-Adhesive dressing be used with AQUACEL or KALTOSTAT dressing.
Change the dressing when clinically indicated or as the softened area approaches the edge of the dressing. The dressing may be left in place for up to 7 days.
Carefully lift the dressing away from the wound.
Comfeel Plus Contour Dressing | |
Wafer: | 24 in2, 42 in2; A6235 |
Comfeel Plus Pressure Relief Dressing | |
Wafer: | 3″ butterfly, 4″ round; A6237 |
6″ round; A6238 | |
Comfeel Plus Sacral Dressing | |
Wafer: | 7″ × 8″; A6239 |
 |
Contraindicated if a wound becomes infected
Must be removed before radiation therapy
Rinse the wound with Sea-Clens or normal saline solution. Gently pat dry the skin around the wound.
Choose a dressing that allows for 3/8″ to 1″ (1 to 2.5 cm) overlap of the wound.
Remove the protective paper from the center of the dressing, and place the dressing on the wound.
Remove the protective paper from the wings, and gently press the wings one at a time to ensure that the dressing adheres to the skin.
Remove the number of foam rings with orange print to get a foam-free area ¾″ to 1″ (1 to 2.5 cm) larger than the wound.
Remove the protective paper from the dressing, and roll the dressing on from one side.
Remove the protective paper from the microporous tape, and gently apply the tape to the skin.
Remove the protective paper from the center of the dressing.
Spread the gluteal fold, place the dressing’s narrow end into the deepest depression of the gluteal fold, and secure it in place. Ensure that the wound has 1″ (2.5 cm) of intact periwound skin and that the dressing adheres to the skin.
Remove the second protective paper from the dressing, and secure the dressing in place.
As Comfeel dressings absorb wound exudate, they turn white or lighten. Change the dressing when the color-change indicator spreads to 3/8″ (1 cm) from the border.
In case of leakage or nonadherence, change the dressing immediately.
Although Comfeel dressings are odorproof, the wound itself may have a characteristic odor. This is normal, and the odor should resolve once the wound is rinsed. If the odor persists, contact a physician.
Comfeel Plus Ulcer Dressing | |
Wafer: | 1½″ × 2½″, 4″ × 4″; A6234 |
6″ × 6″; A6235 | |
8″ × 8″; A6236 | |
Comfeel Ulcer Care Dressing | |
Wafer: | 1½″ × 2½″, 4″ × 4″; A6234 |
6″ × 6″; A6235 | |
Comfeel Plus Transparent Dressing | |
Wafer: | 2″ × 2¾″, 4″ × 4″; A6234 |
3½″ × 5½″, 6″ × 6″; A6235 | |
 |
Contraindicated if a wound becomes infected
Must be removed before radiation therapy
Rinse the wound with Sea-Clens or normal saline solution. Gently pat dry the skin around the wound.
Choose a dressing that allows for 3/8″ to 1″ (1 to 2.5 cm) overlap of the wound.
Use the handles on the dressing to ensure aseptic application. Remove the protective paper.
Place the adhesive side to the wound. Remove the handle.
As Comfeel dressings absorb wound exudate, a gel forms. When the gel reaches the upper film surface of the dressing, it turns white or lightens. Change the dressing when the white gel spreads to 3/8″ (1 cm) from the border.
In case of leakage or nonadherence, change the dressing immediately.
Although Comfeel dressings are odorproof, the wound itself may have a characteristic odor. This is normal, and the odor should resolve once the wound is rinsed. If the odor persists, contact a physician.
DermaFilm HD | |
Film: | 4″ × 4″; A6234 |
DermaFilm Thin | |
Film: | 2″ × 2″, 4″ × 4″; A6234 Sacral |
3″ × 5″ (heel) | |
 |
Contraindicated for third-degree burns
Contraindicated for clinically infected wounds
Contraindicated for ulcers involving muscle, tendon, or bone
Clean the wound area, and dry the periwound skin.
Choose a dressing that overlaps the wound by at least 1″ (2.5 cm). Apply the dressing without stretching it.
Press the dressing gently around the perimeter, forming it to the wound site.
Change the dressing when the exudate extends to the edges. Dressing may be left in place for up to 7 days.
Press down on the skin, and carefully lift an edge of the dressing. Continue lifting around the dressing until all edges are free.
Clean the wound area again.
Sterile dressing: | 2.5″ × 2.5″ dressing plus |
¾″ adhesive border, 4″ × 4″ dressing plus | |
¾″ adhesive border, 4″ × 5″ dressing plus | |
1″ adhesive border; A6237 | |
6″ × 6″ plus 1″ adhesive border, 6″ × 7″ | |
plus 1″ adhesive border; A6238 |
 |
Contraindicated for patients with sensitivity to the dressing or its components
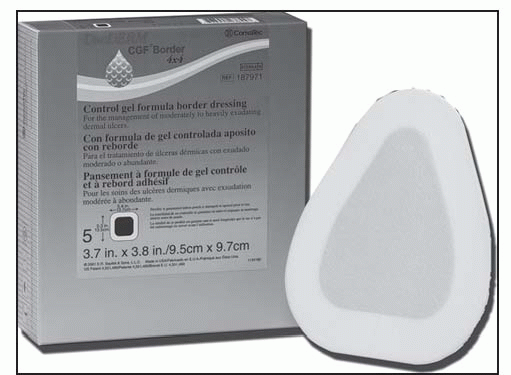
Dressing is sterile; handle appropriately.
Clean the wound according to facility guidelines, and dry the surrounding skin to ensure that it’s grease-free.
Before applying the dressing, remove eschar that’s particularly thick or fused to the wound margins.
Choose a dressing size that’s at least 1¼″ (3 cm) larger than the wound margins.
Remove only the top backing paper.
Apply the dressing over the wound. Smooth into place, especially at the edges of the center adhesive. Note: The triangle-shaped dressing can be applied in several directions, depending on the location of the ulcer. For sacral ulcers, fold the dressing in half lengthwise to make it easy to apply in the sacral fold.
Fold back the border, and remove the release papers; press the borders into place. Additional taping isn’t required.
Obtain a bacterial culture of the site if infection develops, and start appropriate medical treatment as ordered. Continue using the dressing as directed by the primary care provider.
Leave the dressing in place for up to 7 days unless it’s uncomfortable or leaking, or infection develops.
Press down on the skin, and carefully lift an edge of the dressing. Continue lifting around the dressing until all edges are free.
The wound should be cleaned at each dressing change. (It’s unnecessary to remove all residual dressing material from the surrounding skin.)
Not for use on individuals who are sensitive to or who have had an allergic reaction to the dressing or its components
Choose a dressing size to ensure that the dressing is 1½″ (3 cm) larger than the wound area.
Remove the release paper from the back, being careful to minimize finger contact with the adhesive surface.
Hold the dressing over the wound, and line up the center of the dressing with the center of the wound. Place the dressing directly over the wound.
For difficult-to-dress areas, such as heels or the sacrum, a supplementary securing device, such as tape, may be required.
Discard any unused portion of the product after dressing the wound.
Dressing may remain in place up to 7 days. The dressing should be changed when clinically indicated or when strike-through occurs. The wound should be cleaned at each dressing change.
Press down gently on the skin, and carefully lift one corner of the dressing until it no longer adheres. Continue until all edges are free.
DuoDERM Extra Thin Spots: | |
1¼ × 1½″; A6234 | |
DuoDERM ExtraThin Dressing: | |
2″ × 4″, 2″ × 8″, 3″ × 3″, 4″ × 4″; A6234 | |
4″ × 6″, 6″ × 6″; A6235 | |
6″ × 7″ triangle; A6235 | |
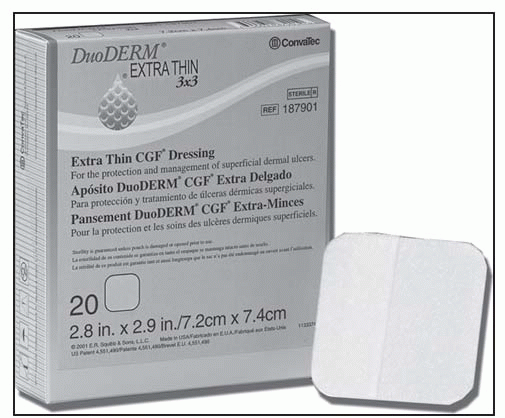 |
Not for use on individuals who are sensitive to or who have had an allergic reaction to the dressings or their components
Dressing is sterile; handle appropriately.
Clean the wound and dry the surrounding skin to ensure that it’s grease-free.
Choose a dressing size that extends beyond the wound margin at least 1¼″ (3 cm).
Minimize finger contact with the adhesive surface.
Apply in a rolling motion; avoid stretching.
Smooth into place, especially around the edges.
Use tape to secure the edges, if necessary.
For a heel or elbow, cut a slit about one third across each side of the dressing to make application easier.
For a sacral ulcer, press the dressing into the anal fold. Depending on the location and depth of the ulcer, the triangle-shaped dressing can be applied in different directions.
Obtain a bacterial culture of the wound site if infection develops, and start appropriate medical treatment, as ordered. Continue using the dressing as directed by the physician. Using an occlusive dressing in the presence of necrotic material may initially increase wound size and depth when the necrotic debris is cleaned away.
Leave the dressing in place for up to 7 days unless it’s uncomfortable or leaking or infection develops.
Press down on the skin, and carefully lift an edge of the dressing. Continue lifting around the dressing until all edges are free.
The wound should be cleaned at each dressing change. (It’s unnecessary to remove all residual dressing material from the surrounding skin.)
Shapes: | 4.5″ × 7.5″ (oval); A6235 |
8″ × 9″ (sacral); A6236 | |
7.5″ × 7.8″ (heel); A6235 | |
6″ × 7″, 8″ × 9″; A6238 | |
Squares: | 4″ × 4″; A6237 |
5.5″ × 5.5″, 8″ × 8″; A6238 |
 |
Not for use on patients who are sensitive to or who have had an allergic reaction to the dressing or its components
Clean the wound surface and surrounding skin with SAF-Clens AF Dermal Wound Cleanser or normal saline solution, and dry the surrounding skin.
Debride if necessary.
Choose a dressing size and shape to ensure that the dressing is 1½″ (3 cm) larger than the wound area.
Hold the dressing by its corner, and pull back the release paper about halfway.
Apply the dressing from the outside edge toward the wound, completely removing the paper backing.
Mold the entire dressing gently but firmly into place.
To remove the dressing, gently press down on the skin with one hand.
Carefully peel up one edge of the dressing with the other hand.
Continue until all edges are free.
Exuderm OdorShield | |
Wafer: | 2″ × 2″, 4″ × 4″; A6234 |
6″ × 6″; A6235 | |
8″ × 8″; A6236 | |
Sacral: | 3.6″ × 4″; A6234 |
6″ × 5″; A6235 | |
Exuderm LP | |
Wafer: | 4″ × 4″; A6234 |
6″ × 6″; A6235 | |
Exuderm RCD | |
Wafer: | 4″ × 4″; A6234 |
6″ × 6″; A6235 | |
8″ × 8″; A6236 | |
 |
Contraindicated for third-degree burns