Chapter 26 HIV-associated tuberculosis
Introduction
Over the last three decades, tuberculosis (TB) and HIV epidemics have become inextricably connected in a bidirectional interaction, often seen as a deadly association or “the cursed duet” [1]. TB remains a major cause of morbidity and mortality in HIV-infected individuals, especially in countries with a high burden of both infections. The HIV pandemic has changed TB epidemiology, natural history, and pathogenesis, affecting its clinical and radiographic presentation, diagnosis, treatment, and prognosis.
In general, people living with HIV are 20 to 37 times more likely to develop TB disease during their lifetimes than HIV-uninfected individuals [2]. Patients with advanced HIV disease who develop TB are more likely to manifest atypical radiographic findings and develop extrapulmonary and disseminated disease. The diagnosis of TB in HIV-infected patients is more difficult, and the treatment is often complicated by drug interactions, cumulative toxicities and decreased efficacy. Furthermore, mortality rates are remarkably higher. Among individuals treated for TB, HIV-infected individuals have higher rates of recurrent TB than those who are HIV-uninfected. Conversely, active TB is associated with increased viral replication in HIV-infected patients, contributing to HIV progression and shortening survival in dually-infected patients.
Epidemiology
TB is a major global health problem, ranking as the leading cause of death from an infectious agent and the seventh cause of death in the world [3]. The combined burden of disease caused by HIV and TB is daunting. More than 33 million people were living with HIV at the end of 2009, and the death toll from HIV/AIDS in the same year was 1.8 million. It is estimated that one-third of the HIV-infected population is co-infected with Mycobacterium tuberculosis, the majority harboring latent TB infection.
In 2009, there were 9.4 million incident cases of TB globally, equivalent to 137 cases per 100,000 population. TB prevalence was estimated at 14 million cases, equivalent to 200/100,000 [2]. Most of the estimated number of cases in 2009 occurred in Asia (55%) and Africa (30%), and smaller proportions of cases occurred in the Eastern Mediterranean region (7%), the European region (4%), and the Americas (3%). Approximately 80% of all estimated cases worldwide were concentrated in 22 high–burden countries (HBCs), among which India alone accounts for an estimated one-fifth (21%), and China and India combined account for 35% of all TB cases worldwide (Fig. 26.1A).
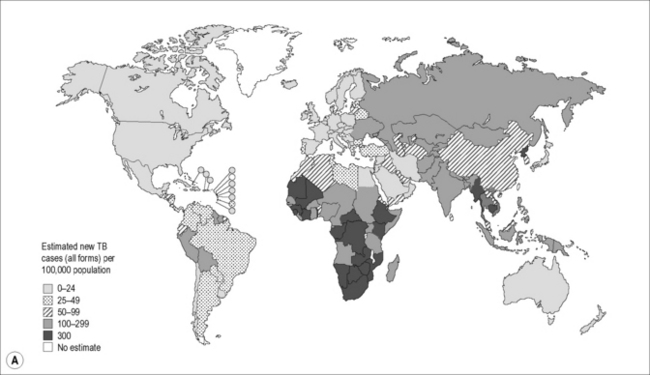
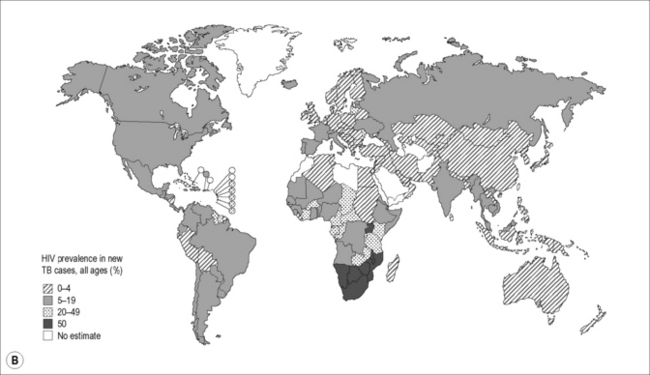
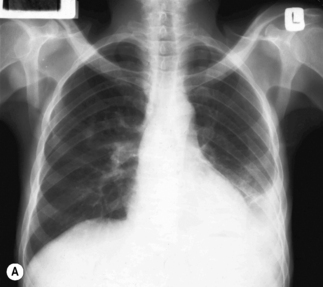
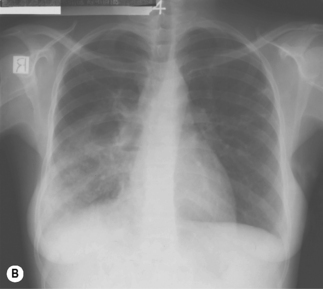
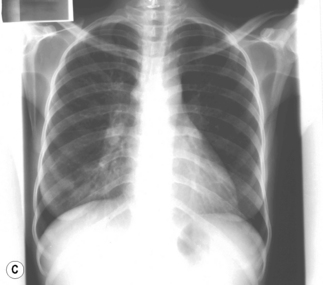
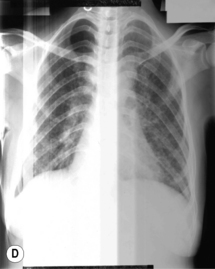
Figure 26.1 (A) Estimated TB incidence rates by country in 2009. (B) Estimated HIV prevalence in new TB cases in 2009.
Reproduced from World Health Organization. Global Tuberculosis Control. [online]. 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241564069_eng.pdf
Of the 9.4 million incident cases in 2009, an estimated 1.1 million (12%) were HIV-infected, with approximately 80% of the TB-HIV cases occurring in Africa. In some southern and eastern African countries, more than 50% of TB patients are co-infected with HIV (Fig. 26.1B). Approximately 1.7 million people died of TB in 2009, including an estimated 0.4 million people with HIV, amounting to about one in four of the deaths occurring among HIV-infected individuals.
TB drug resistance, including multi-drug resistant TB (MDR-TB) and extensively-drug resistant TB (XDR-TB) is an increasing global problem. MDR-TB is defined as resistance to at least isoniazid and rifampicin, and XDR-TB is defined as an MDR isolate that is also resistant to any fluoroquinolone and at least one of the injectable second-line drugs (capreomycin, amikacin and kanamycin). There were an estimated 440,000 cases of MDR-TB in 2008, of which 85% occurred in 27 high MDR-TB burden countries; 150,000 deaths from MDR-TB occurred in 2008. The global epidemiology of drug-resistant TB in HIV-infected persons is not known. A systematic review that included 32 studies from 17 countries failed to demonstrate an overall association between MDR-TB and HIV infection [4]. However, HIV is a potent risk factor for institutional outbreaks of MDR-TB. In outbreak settings the case mortality rate has been extremely high.
Pathogenesis and Natural History
TB stages are conventionally viewed as “primary TB,” “progressive primary TB,” “latent TB,” and “secondary or post-primary TB”. There is, however, evidence supporting the paradigm that the interaction between M. tuberculosis and the human host represents a continuum of immune responses, pathologic manifestations, mycobacterial metabolic activity and clinical disease [5]. Nonetheless, this chapter will utilize the classical presentation for simplicity.
Transmission of M. tuberculosis
Environmental characteristics such as ventilation, humidity or presence of UV light influence the likelihood of transmission. The likelihood of TB transmission also depends on the infectiousness of the source case as measured by the cough strength [6] and the grade of acid-fast bacilli (AFB) sputum smear results. Patients with cavitary lesions and intensely positive sputum smears have a higher risk of TB transmission. Bacterial factors such as virulence and viability also influence M. tuberculosis transmission, as evidenced by the Beijing strain family of M. tuberculosis that has been associated with increased transmission, dissemination and outbreaks. Transmission of MDR-TB is comparable to that of drug-susceptible M. tuberculosis.
In general, TB transmission occurs as a consequence of household exposure, the prolonged and frequent contact with an active TB patient being much more likely to transmit M. tuberculosis than a brief contact. This pattern is observed in both low- and high-prevalence countries, irrespective of HIV status and can be demonstrated by traditional epidemiological studies and confirmed by molecular approaches [7, 8]. Occasionally, M. tuberculosis transmission has been reported after casual contact with an infectious case. Transmission after brief exposure has been linked more often to outbreaks in shelters, nursing homes, hospitals, prisons or air travel. In such cases, transmission may be related to increased virulence of the involved strain, environmental factors or patient characteristics. There is, in fact, evidence that some patients with pulmonary TB are “super-transmitters.”
Remarkably, only about one-half of the household contacts of active TB patients become infected [9], suggesting that in addition to the type of exposure, host-related factors may influence susceptibility to M. tuberculosis. The existence of innate immunity to TB is a certainty, although the immunologic mechanisms that render some populations susceptible and other resistant to TB remain largely uncharacterized. Several studies have demonstrated the association of various human leukocyte antigens (HLA) with disease susceptibility in different ethnic populations [10, 11]. Genetic susceptibility to TB has also been associated with polymorphisms in the human SLC11A1 (formerly NRAMP1) gene, some toll-like receptor (TLR) genes, the genes for the vitamin D receptor and components of the interferon (IFN) gamma-signaling pathways.
The effect of HIV on M. tuberculosis transmission has been studied extensively with contradictory results. A meta-analysis concluded that HIV infection does not significantly increase the risk of M. tuberculosis transmission, as patients with HIV-1 infection and TB are no more infectious than HIV–uninfected patients with TB [12].
TB disease
In HIV-infected individuals, post-primary TB most commonly develops through the endogenous reactivation of the small foci of hematogenous dissemination occurring in the course of primary infection, as in HIV-uninfected populations. In both HIV-infected and uninfected individuals, the most common site of TB reactivation is the lung. Extrapulmonary TB, which may occur in any organ, is more common in HIV-infected patients. In contrast to HIV-uninfected individuals, active TB occurring as a result of TB re-infection, proved through restriction fragment length polymorphism (RFLP) analysis of strains, has been observed frequently in HIV-infected patients, especially in countries where the prevalence of TB is high [13, 14].
In HIV-uninfected individuals, the lifetime risk of developing TB disease by reactivating latent TB infection is approximately 5–10%. In HIV-infected individuals, this risk increases to 5–15% annually, rising as immune deficiency worsens. The increased risk is manifest even in the absence of immunodeficiency. In South African gold miners, the risk of active TB was increased two- to threefold within the first 2 years of HIV infection despite the absence of significant CD4 cell depletion [15]. In general, the risk of TB progression correlates with the CD4 count. In an African cohort, the TB incidence rate was 17.5 cases/100 person-years in HIV-infected patients with CD4 counts <200/mm3 versus 3.6 cases/100 person-years for CD4 counts >350/mm3 [16].
Immunologic aspects
Besides expressing traditional phagocytic receptors for antibody and complement, macrophages and dendritic cells also express Toll-like receptors (TLRs) that recognize conserved antigens expressed on pathogens. Binding of TLRs to these pathogen-specific ligands initiates a signal transduction pathway in the host cell that culminates in the activation of NFκB and the induction of cytokines and chemokines that are crucial to eliciting the adaptive immune response against M. tuberculosis. The sequence of the initial immune events following interaction of M. tuberculosis with TLRs and other receptors is not completely understood. Nevertheless, it is clear that in the vast majority of individuals, the interaction culminates in the development of a protective Th1 dominant immune response [17]. Th1 lymphocytes are characterized by expression of IL-2 and IFN-γ.
Impact of TB infection on HIV
Immunologic activation induced by M. tuberculosis is associated with an overproduction of cytokines, such as TNF that increases HIV replication in latently-infected cells. One study demonstrated a 5- to 160-fold increase in viral replication during the acute phase of untreated TB [18]. It is also observed that active TB sometimes induces a transient CD4 T cell depletion, which may contribute to HIV progression.
Clinical Manifestations
Primary tuberculosis
Secondary tuberculosis
Pulmonary tuberculosis
Cough, fever, fatigue, weight loss, and night sweats are the most frequent symptoms of pulmonary TB used in clinical algorithms for TB screening in both HIV-infected and HIV-uninfected individuals. Several studies were conducted to determine the performance of these symptoms in predicting the diagnosis of TB in HIV-infected patients. Screening algorithms that combine multiple symptoms have demonstrated higher sensitivity, but lower specificity. A Southeast Asian study of HIV-infected individuals has shown that the presence of cough, fatigue, fever or weight loss in the previous 4 weeks had a sensitivity greater than 70% for each of the individual symptoms, with relatively lower specificities. However, the performance of clinical indicators was greatly increased if a combination of these symptoms was used for TB screening [19]. Although the presence of cough for 2–3 weeks has traditionally served as a criterion for identifying the TB suspects, this duration was found to have a relatively low (22–33%) sensitivity in this study, whereas a cough of any duration in the previous 4 weeks had a sensitivity greater than 70%. The combination of cough of any duration, fever of any duration and night sweats lasting 3 or more weeks in the preceding 4 weeks was 93% sensitive for TB and had a negative predictive value of 97%. A subsequent WHO meta-analysis of 12 studies that included more than 8,000 HIV-infected patients concluded that the absence of current cough, fever, night sweats, and weight loss (all inclusive) had a negative predictive value of ~98% in a setting with TB prevalence >5% [20].
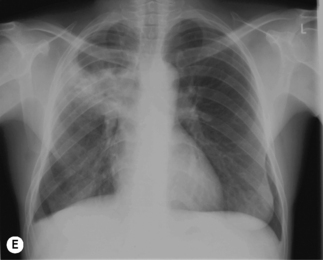
In TB associated with HIV infection, radiographic manifestations correlate with the level of immunosuppression. In patients with CD4 counts >350 cells/mm3, TB presents with classical radiographic findings of reactivation disease. In patients with fewer CD4 cells, the likelihood of atypical radiographic findings increases, and up to 20% of patients may have a normal or near-normal chest radiograph. In a study of radiographic patterns of TB in HIV-infected patients, diffused or localized infiltration were more frequent, as well as hilar or mediastinal lymphadenopathy. Pleural disease, cavitation, and normal radiography were the least common findings [21]. However, studies conducted in Africa demonstrated a greater frequency of cavitation in HIV-infected patients with TB, possibly related to the high incidence of TB in this region. Several radiographical forms of pulmonary TB are illustrated in Fig. 26.2.
Extrapulmonary tuberculosis
Pleural TB
Pleural TB occurs by direct extension from an adjacent sub-pleural pulmonary focus or through hematogenous seeding. Typical presentation is the abrupt onset of fever, pleuritic chest pain, and cough. Occasionally there is an insidious presentation with fever, weight loss, and malaise. If the pleural effusion is large enough, there may be shortness of breath. Physical examination shows dullness to percussion and decreased breath sounds. Egophony is a helpful sign if present. Chest radiograph typically shows unilateral pleural effusion more frequently in the right hemithorax. Bilateral disease is seen in 10% of cases. Pleural fluid analysis demonstrates high protein concentration, low glucose concentration, and lymphocytosis. The presence of >5% mesothelial cells in the pleural fluid usually argues against a diagnosis of TB pleural effusion, as the chronic pleural inflammation is thought to prevent the exfoliation of mesothelial cells in the pleural cavity. The AFB smear is positive in less than 10% of cases, and the yield of culture has been less than 30% in most series, with a range between 12% and 70%. In patients co-infected with HIV, the yield of pleural fluid and pleural biopsy culture may be higher than in HIV-uninfected individuals with TB pleurisy [22]. Pleural biopsy may show granulomas in approximately half of the cases, and the yield is higher on pleural biopsy culture. An elevated concentration (>70 U/L) of pleural fluid adenosine deaminase (ADA) may suggest the diagnosis of TB.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree