• Explain the key concepts of basic human genetics. • Discuss the purpose, key findings, and potential outcomes of the Human Genome Project. • Describe expanded roles for nurses in genetics and genetic counseling. • Examine the ethical dimensions of genetic screening. • Discuss the current status of gene therapy (gene transfer). • Summarize the process of fertilization. • Describe the development, structure, and functions of the placenta. • Describe the composition and functions of the amniotic fluid. • Identify three organs or tissues arising from each of the three primary germ layers. • Summarize the significant changes in growth and development of the embryo and fetus. • Identify the potential effects of teratogens during vulnerable periods of embryonic and fetal development. Additional related content can be found on the companion website at http://evolve.elsevier.com/Lowdermilk/Maternity/ • Animation: Fertilization and Implantation • Animation: First Trimester, Fetal Development • Animation: Male Reproductive Ducts • Animation: Male Accessory Sex Glands • Animation: Male External Genitalia • Animation: Maternal and Fetal Circulation • Animation: Oogenesis and Meiosis • Animation: Second Trimester, Fetal Development • Animation: Third Trimester, Fetal Development • Critical Thinking Exercise: Genetic Counseling • Nursing Care Plan: The Family with an Infant Who Has Down Syndrome Some disorders appear more often in ethnic groups. Examples include Tay-Sachs disease in Ashkenazi Jews, French Canadians of the Eastern St. Lawrence River valley area of Quebec, Cajuns from Louisiana, and the Amish in Pennsylvania; beta thalassemia in Mediterranean, Middle Eastern, Transcaucasus, Central Asian, Indian, and Far Eastern groups, as well as those of African heritage; sickle cell anemia in African-Americans; alpha thalassemia in those from Southeast Asia, South China, the Philippine Islands, Thailand, Greece, and Cyprus; lactase deficiency in adult Chinese and Thailanders; neural tube defects in Irish, Scots, and Welsh; phenylketonuria (PKU) in Irish, Scots, Scandinavians, Icelanders, and Polish; cystic fibrosis (CF) in Caucasians, Ashkenazi Jews, and Hispanics; and Niemann-Pick disease type A, in Ashkenazi Jews (Hamilton & Wynshaw-Boris, 2009; Solomon, Jack, & Feero, 2008) (Cultural Considerations box). Genetic disorders span every clinical practice specialty and site, including school, clinic, office, hospital, mental health agency, and community health settings. Because the potential impact on families and the community is significant (Box 5-1), genetic information, technology, and testing must be integrated into health care services, and genetics must be integrated into nursing education and practice. Nurses are usually the ones who provide follow-up care and maintain contact with the patients. Community health nurses can identify groups within populations that are at high risk for illness, as well as provide care to individuals, families, and groups. These nurses provide a vital link in follow-up for newborns who may need newborn screening (http://genes-r-us.uthscsa.edu). In 2005 a panel of more than 50 nursing leaders from clinical, research, and academic settings developed and came to consensus on a document, “Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics.” The competencies in the document reflect the minimal amount of genetic and genomic competency expected of all nurses. The competencies are not intended to replace or recreate current standards of practice. The document is available online at www.nursingworld.org/MainMenuCategories/EthicsStandards/Genetics_1.aspx. Examples of competencies in the professional practice domain include that the registered nurse: • Demonstrates an understanding of the relationship of genetics and genomics to health, prevention, screening, diagnostics, prognostics, selection of treatment, and monitoring of treatment effectiveness • Demonstrates ability to elicit a minimum of three-generation family health history information • Constructs a pedigree from collected family history information using standardized symbols and terminology • Collects personal, health, and developmental histories that consider genetic, environmental, and genomic influences and risks • Assesses patients’ knowledge, perceptions, and responses to genetic and genomic information • Develops a plan of care that incorporates genetic and genomic assessment information Genetics-related activities that all nurses should be able to provide are further delineated in Genetics/Genomics Nursing: Scope and Standards of Practice (2nd ed.) (International Society of Nurses in Genetics [ISONG] and American Nurses Association [ANA], 2006). This document includes standards and levels of practice for genetics nursing that were established cooperatively by ISONG and ANA. These activities are not limited to particular practice settings, nor are they limited to specific specialty areas. Determining whether a heritable disorder exists in a couple or in anyone in either of their families is standard practice in obstetrics. The goal of screening is to detect or define risk for disease in low risk populations and identify those for whom diagnostic testing may be appropriate. Obtaining a complete three-generation medical history that includes ethnicity information is the best genetic “test” applicable to preconception care (Solomon Jack, & Feero, 2008). The U.S. Surgeon General Family History Initiative has posted My Family Health Portrait at https://familyhistory.hhs.gov/fhh-web/home.action. Nurses can recommend to their patients that they complete a family history using this website. In addition, nurses can obtain a genetic history using a questionnaire or a checklist such as the one in Fig. 5-1. Learning about conditions that might affect the pregnancy in the preconception period is ideal (Solomon et al., 2008). Individuals can then make informed reproductive decisions that might include adoption, surrogacy, or use of donor sperm. However, most genetic testing is offered prenatally so as to identify genetic disorders in fetuses (Wapner, Jenkins, & Khalek, 2009). When an affected fetus is identified, parents can be prepared for birth of such an infant. Termination of the pregnancy is also an option. Other requests for genetic testing occur for sex selection or for late-onset disorders. An ethic of social responsibility should guide genetic counselors in their interactions with patients while recognizing that people make their choices by integrating personal values and beliefs with their new knowledge of genetic risk and medical treatments. Other ethical issues relate to autonomy, privacy, and confidentiality. Should genetic testing be performed when no treatment is available for the disease? When should family members at risk for inherited diseases be warned? When should presymptomatic testing be performed? Some who might benefit from genetic testing choose not to have it, fearing discrimination based on the risk of a genetic disorder. Several states have prohibitions against insurance discrimination; other states are expected to follow their lead. Until guidelines for genetic testing are created, caution should be exercised. The benefits of testing should be weighed carefully against the potential for harm. The American Academy of Pediatrics (2001) and the Canadian College of Medical Geneticists (2003) recommend against genetic testing of children for disorders that have a late-onset and for which no treatment exists. Preimplantation genetic screening (PGS) is available in a limited number of centers. In this procedure, embryos are tested before implantation by in vitro fertilization (IVF) (Wapner et al., 2009). PGS has the potential to eliminate specific disorders in pregnancies conceived by IVF. Work is ongoing to test fetal cells and nucleic acid retrieved from the maternal blood samples as means of noninvasive prenatal diagnosis (Wapner et al.). The Human Genome Project was a publicly funded international effort coordinated by the National Institutes of Health (NIH) and the U.S. Department of Energy. Not only was the Human Genome Project responsible for a long list of amazing genetics discoveries, but it also stimulated and facilitated the work of thousands of scientists worldwide. Within 24 hours after a piece of DNA had been sequenced by Human Genome Project scientists the results were posted on a public database (www.ncbi.nlm.nih.gov/genome/guide/human); no restrictions on its use or redistribution have been enacted. Two key findings from initial efforts to sequence and analyze the human genome are that (1) all human beings are 99.9% identical at the DNA level, and (2) approximately 30,000 to 40,000 genes (pieces or sequences of DNA that contain information needed to make proteins) make up the human genome (International Human Genome Sequencing Consortium, 2001). The finding that human beings are 99.9% identical at the DNA level should help to discourage the use of science as a justification for drawing precise racial boundaries around certain groups of people. The vast majority of the 0.1% genetic variations are found within and not among populations. The finding that humans have 30,000 to 40,000 genes, which is only twice as many as roundworms (18,000) and flies (13,000), was unexpected. Scientists had estimated the human genome contained 80,000 to 150,000 genes. The assumption was that humans are more evolved and more highly sophisticated than other species because they have more genes. Initial efforts to sequence and analyze the human genome have proven invaluable in the identification of genes involved in disease and in the development of genetic tests. More than 100 genes involved in diseases such as Huntington disease (HD), breast cancer, colon cancer, Alzheimer disease, achondroplasia, and CF have been identified. Genetic tests for 1672 inherited conditions are commercially available; of these, 1379 are clinical tests and 293 are research tests (www.genetics.org). Most of the genetic tests now being offered in clinical practice are tests for single-gene disorders in patients with clinical symptoms or who have a family history of a genetic disease. Some of these genetic tests are prenatal tests or tests used to identify the genetic status of a pregnancy at risk for a genetic condition. Current prenatal testing options include maternal serum screening (a blood test used to see if a pregnant woman is at increased risk for carrying a fetus with a neural tube defect or a chromosomal abnormality such as Down syndrome) and invasive procedures (amniocentesis and chorionic villus sampling) (see Chapter 19). Other tests are carrier screening tests, which are used to identify individuals who have a gene mutation for a genetic condition but do not show symptoms of the condition because it is a condition that is inherited in an autosomal recessive form (e.g., CF, sickle cell disease, and Tay-Sachs disease) (Peach & Hopkin, 2007). The incidence of chromosomal aberrations is estimated to be 0.6% in newborns. Approximately 62% of miscarriages and 10% of stillbirths and perinatal deaths are caused by chromosomal abnormalities (Hamilton & Wynshaw-Boris, 2009). Errors resulting in chromosomal abnormalities can occur in mitosis or meiosis. These errors occur in either the autosomes or the sex chromosomes. Even without the presence of obvious structural malformations, small deviations in chromosomes can cause problems in fetal development. The pictorial analysis of the number, form, and size of an individual’s chromosomes is known as a karyotype. Cells from any nucleated, replicating body tissue (except red blood cells, nerves, or muscles) can be used. The most commonly used tissues are white blood cells and fetal cells in amniotic fluid. The cells are grown in a culture and arrested when they are in metaphase, and then the cells are dropped onto a slide. This process breaks the cell membranes and spreads the chromosomes, making them easier to visualize. The cells are stained with special stains (e.g., Giemsa stain) that create striping or “banding” patterns. These patterns aid in the analysis because they are consistent from person to person. Once the chromosome spreads are photographed or scanned by a computer, they are cut out and arranged in a specific numeric order according to their length and shape. The chromosomes are numbered from largest to smallest, 1 to 22, and the sex chromosomes are designated by the letter X or Y. Each chromosome is divided into two “arms” designated by p (short arm) and q (long arm). A female karyotype is designated as 46, XX and a male karyotype is designated as 46, XY. Fig. 5-2 illustrates the chromosomes in a body cell and a karyotype. Karyotypes can be used to determine the sex of a child and the presence of any gross chromosomal abnormalities. The most common trisomal abnormality is Down syndrome, or trisomy 21 (47, XX+21, female with Down syndrome; or 47, XY+21, male with Down syndrome). Although the risk of having a child with Down syndrome increases with maternal age (incidence is approximately 1 in 1200 for a 25-year-old woman, 1 in 350 for a 35-year-old woman, and 1 in 30 for a 45-year-old woman), children with Down syndrome can be born to mothers of any age. Eighty percent of children with Down syndrome are born to mothers younger than 35 years (National Down Syndrome Society, 2006) (Nursing Care Plan).
Genetics, Conception, and Fetal Development
Web Resources
![]()
Genetics

Genetics and Nursing
Genetic History-Taking and Genetic Counseling Services

Ethical Considerations
The Human Genome Project
Genetic testing
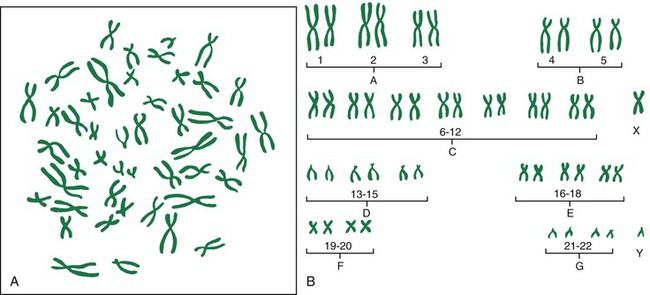
Chromosomal Abnormalities
Autosomal abnormalities
Abnormalities of chromosome number.

Genetics, Conception, and Fetal Development
Get Clinical Tree app for offline access


