Coitus
In the male
Fraction
Dominant Gland
Particularly Rich in
Initial
Prostate
Acid phosphatase
Mid
Vas deferens
Sperm
Late
Seminal vesicles
Fructose
In the female
The gametes
Male gametes
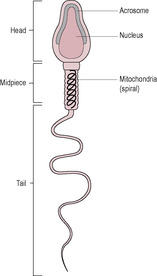
Fig. 6.1
Type of Abnormality
Description
Possible Causes
Azoospermia (aspermia)
No sperm present within the ejaculate
Primary testicular failure; blockage to the vas deferens, that is, infection or trauma
Oligozoospermia (oligospermia)
Reduced numbers of sperm in the ejaculate (low sperm count)
Gonadotrophin insufficiency; drugs (social and medical, alcohol, toxins, etc.)
Idiopathic oligospermia
Low sperm count but physiological parameters normal
Unexplained
Teratozoospermia (teratospermia)
Abnormal morphology, for example, giant heads, double tails
Genetic, toxins, viral infection
Asthenospermia
Reduced (or lack of) mobility
Toxins, infection
Sperm agglutination
Sperm clump together in groups
Infection, Production of antigens against sperm (autoimmune response)
Sperm competition
Female gametes
The fertile window
Sex of the zygote
Stages of fertilization
Gamete motility and sperm deposition
Capacitation
Access to the oocyte
Binding to the zona pellucida
The acrosome reaction
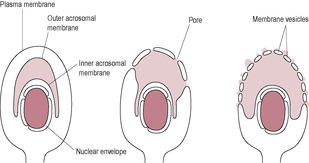
Fig. 6.2
Gamete fusion
The cortical reaction and block to polyspermy
The zona reaction
Events leading to the first mitotic division
Possible benefits of arrested meiosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Fertilization
Zara and James have attended the local hospital for the 12-week scan and have been informed that there is now only one baby present in the uterus. It would appear that one of the sacs, observed at Zara’s earlier scans, had failed to develop further and the ultrasound scan could no longer detect any evidence of the sac’s remnants. Zara and James are understandably upset over the loss of one of their babies but are reassured that the other baby has an extremely low risk of having Down’s syndrome, is growing well and appears normal.
• How would you explain to Zara and James what were the possible reasons for one of the embryos failing to develop and is it more likely to occur in heterozygous or homozygous twins?
• How common is the loss of a twin in early pregnancy and what reasons could explain why this happens?
Mammalian fertilization occurs within the female reproductive tract. Less than one in a million sperm, or spermatozoa, reach the oocyte. The sperm are deposited in the vagina during intercourse (coitus) and then make the long journey through the female reproductive tract (equal to about 10000 times their own length). The hazards and challenges imposed on the successful fertilization are thought to help ensure that the individual sperm that actually fertilizes the oocyte is strong and healthy. There is extensive and intricate crosstalk between the oocyte and the fertilizing sperm, which leads to activation of the egg and sperm head decondensation. Following fertilization, the female and male pronuclei form, syngamy occurs and the zygote undergoes the first cleavage divisions while travelling through the uterine tube to the uterus where it implants in the uterine wall.
Coitus in humans lasts on average 4 min, which is quite long compared to our closest animal cousin, the chimpanzee, which averages 8 s (De Waal, 1995). Masters and Johnson (described in Levin, 1998) described a four-phase model for sexual responses in humans. This is known as the EPOR model:
• E: excitement phase, when stimuli increase sexual arousal or tension.
• P: plateau phase, when arousal becomes intense; if the level of stimulation is inadequate, arousal subsides and there is no further progression to the next phase.
• O: orgasmic phase, which is a few seconds of involuntary climax during which sexual tension is relieved, usually accompanied by a wave of profound pleasure.
• R: resolution phase, when sexual arousal is dispersed; in males, it is believed that there is an absolute refractory period in which further sexual arousal and orgasm are impossible.
The principal event necessary for male sexual activity is acquisition and maintenance of penile erection. This is primarily a vascular phenomenon, initiated by neurological controls and facilitated by appropriate psychological and hormonal components. Initial stimulation of the penis can be both psychogenic (from erotic stimuli and sexual fantasy) and tactile (via touch receptors in the penis and perineum) and necessitates autonomic nervous system activity to coordinate increased blood flow into the vascular tissues. Centrally perceived sensual stimuli are relayed via the spinal thoracolumbar erection centre (T11 through L2), and reflex erections, initiated by tactile stimuli to the genital area, activate a reflex arc involving the sacral erection centre (S2 through S4; Dey Shepherd, 2002). Involuntary non-sexual nocturnal erections occur during rapid eye movement sleep. There are three components of erection: increased arterial flow, relaxation of the sinusoidal spaces and venous constriction. In the excitement phase, increased inflow of blood converts the low-volume, low-pressure vasculature to a large-volume, high-pressure system (Andersson and Wagner, 1995).
The arterioles and arteriovenous shunts dilate so there is an increase in blood flow which engorges the erectile vascular tissues (cavernous and spongiosum bodies, see Chapter 2). The corpus spongiosum does not increase in turgor as much as the two corpora cavernosa so the urethra is not compressed. Blood outflow is occluded by compression and constriction of the veins so the sinusoids (blood-filled spaces) enlarge further. This results in hardening and erection of the penis as the blood volume is increased by about 50%.
The control of an erection is by stimulation of parasympathetic nerves, and probably simultaneous inhibition of sympathetic outflow, which reduces arterial smooth muscle tone causing dilatation and increase in blood flow. Many central and peripheral neurotransmitters are involved in mediating the erectile response. Centrally, dopamine, nitric oxide (NO), oxytocin and adrenocorticotrophin (ACTH)/melanocyte-stimulating hormone (α-MSH) seem to have a facilitatory role; serotonin may be either facilitatory or inhibitory, and enkephalins are inhibitory. Peripherally, the balance between vasoconstrictive factors (such as noradrenaline, endothelin and angiotensin) and vasodilatory factors (such as NO, vasoactive intestinal peptide (VIP) and prostanoids) controls the tone of the smooth muscle of the corpora cavernosa and determines the functional state of the penis. Other neurotransmitters implicated in the erectile response are acetylcholine, endothelins and Rho-kinases. NO is considered the most important factor facilitating vasodilatation, thus maximizing blood flow and penile erection; it promotes the generation of cyclic guanosine monophosphate (cGMP) which increases smooth muscle relaxation and inflow of blood. Detumescence (loss of erection) occurs because NO-induced vasodilation abates as cGMP is broken down predominantly by the intracavernosal cGMPphosphodiesterase (cGMP–PDE5). Sildenafil citrate (Viagra) and related oral therapies for erectile dysfunction (ED), such as vardenafil (Levitra) and tadalafil (Cialis), selectively inhibit the breakdown of cGMP by PDE5. Side effects of these drugs may include headache, flushing, rhinitis, urinary tract infections, visual disturbances, diarrhoea and dyspepsia, and they may be contraindicated for men with heart and various other medical conditions.
Descending pathways can be either excitatory (such as those initiated by the perceived attractiveness of the partner) or inhibitory (such as anxiety or guilt). Testosterone, mediated by oestradiol, is required to maintain intrapenile NO synthase levels, which mediate local vasodilatation by increasing NO production. In hypogonadal men, testosterone therapy can restore libido and erectile function. Other hormones, such as prolactin, adrenal steroids and thyroid hormone, contribute to male sexual functioning.
ED (‘impotence’), defined as the inability to achieve and/or to maintain an erection for long enough to permit satisfactory sexual intercourse, can be caused by abnormalities of circulation or of neural inputs affecting the nervous control of erection. ED can result from psychogenic, organic (neurogenic such as spinal trauma, hormonal, vascular, cavernosal or drug induced) or mixed causes. Most cases of ED have an organic origin, mostly vascular disease. Atherosclerosis results in endothelial damage, cellular migration and smooth muscle proliferation influenced by cytokines, thrombosis, growth factors, reactive-oxygen species (ROS) and metabolic changes. Ageing affects NO production by the endothelium. Smoking is a risk factor for ED because it affects the vascular endothelium and nicotine, both increases sympathetic tone leading to smooth muscle contraction in the cavernosal body and decreases activity of the enzyme nitric oxide synthase. Failure of erection can be caused by damage to the spongy bodies, impaired flow in the vessels supplying the penis, drugs that interfere with neurotransmitter action or psychogenic factors. Local atherosclerosis can affect blood flow as can nicotine, which has vasoconstrictive properties.
ED is usually treated with vacuum devices, intraurethral suppositories, intracavernosal injections of vasoactive drugs (such as prostaglandin E1 (PGE1, alprostadil) and papaverine, which increase cAMP levels) and, more recently, with oral antagonists of cGMP–PDE (see above). (However, note that many men do not like or cannot tolerate PDE inhibitors.) Neurogenic ED can also arise from lesions in the nervous system, such as peripheral nerve damage. At the cellular level, alterations in potassium efflux may lead to a state of hypercontraction and lack of erectile response. ED is a common age-related problem so the incidence of ED will increase as longevity increases; ageing is associated with a decline in testosterone which may compromise the oestrogen–androgen crosstalk involved in the control of erection. Diabetes, particularly if coexisting with obesity, is a risk factor for ED and is related to accelerated atherosclerosis, alterations in erectile tissue, neuropathy and changes in hormone levels (Tamler, 2009). Diabetes-associated changes include smooth muscle degeneration, endothelial cell dysfunction, abnormal collagen deposition and high levels of glycosylated end products which reduce NO levels; these result in impaired relaxation of the corpus cavernosum smooth muscle (Sullivan et al., 2002). Medications for hypertension can contribute to ED; β-blockers can cause ‘dry’ ejaculation which is probably due to retrograde ejaculation because the β-blockers are causing bladder neck relaxation.
ED can also be a manifestation of cardiovascular disease; hyperlipidaemia and hypercholesterolaemia affect the production of NO by the vascular endothelium. ED is a symptom not a disease; it may act as a barometer of cardiovascular health and be the first presenting sign of previously undiagnosed hypertension, atherosclerosis and diabetes. Although the incidence of ED is common and increasing, it is underreported, underdiagnosed and undertreated as many men are reluctant to seek help. The condition can have considerable psychological and social impact on the affected man and his partner and their quality of life, causing depression, anxiety and loss of self-esteem.
As the penis becomes erect, the testes increase their blood volume and are drawn up towards the perineum. The dartos muscle contracts so the scrotal skin thickens and contracts. As stimulation proceeds, the plateau phase of emission occurs, where the muscles of the prostate, vas deferens and seminal vesicle undergo coordinated responses that propel spermatozoa and seminal fluid into the urethra. Ejaculation, the orgasmic phase, is the process of ejecting sperm from the urethra following contraction of the urethra smooth muscle. This is accompanied by contraction of the pelvic floor muscles and the accessory muscles including the vesicular urethral sphincter, which prevents retrograde ejaculation into the bladder. Orgasm, or contraction of the muscles, can occur without ejaculation. Oxytocin has a key role in the regulation of erection and, together with endothelin-1, in coordinated contraction of the epididymis and tubules at orgasm (Filippi et al., 2003); oxytocin responsiveness seems to be mediated by oestrogen. The composition of ejaculate changes because the contractions are sequential and there is relatively little mixing of the components (Table 6.1).
Premature or rapid ejaculation is the most common ejaculatory dysfunction and is usually caused by anxiety or emotional stress (Master and Turek, 2001); it is treated with behavioural therapy, pelvic floor muscle exercises, α-adrenoceptor antagonists (‘sympathetic α-blockers’), topical anaesthetics, tricyclic antidepressants and selective serotonin reuptake inhibitors such as fluoxetine and sertraline. There have also been some clinical trials investigating the use of PDE5 inhibitors in the treatment of rapid ejaculation. Neurological lesions, such as spinal cord damage or damage following injury or surgery to the colon or abdomen, can also cause ejaculatory dysfunction; the sympathetic nerves associated with control of sexual functioning can easily be damaged. Other problems, such as diabetes and multiple sclerosis, can cause ejaculatory problems. Drugs, such as medication for hypertension and the common cold, may cause a lack of emission (deposition of the seminal fluid into the posterior or back part of the urethra), resulting in failure of ejaculation. Bladder neck damage in men who have undergone prostate surgery (particularly, transurethral resection of the prostate) can result in retrograde ejaculation. Bloody ejaculation is usually due to haematospermia, a benign, self-limited condition resulting from inflammation of the seminal vesicles, colon or prostate, which is treatable by antibiotics.
Women have similar responses in coitus to those of men. Tactile stimulation of the perineal region and the glans clitoris as well as psychogenic stimuli elicit the response. The corpora of the clitoris and the labia undergo vascular engorgement. Increased blood flow to the vagina increases transudation and vaginal lubrication. The vagina increases in width and length and the uterus is elevated upwards, which lifts the cervical os to produce a ‘tenting effect’. At orgasm, vaginal and uterine contractions increase in intensity. During orgasm, women may expel fluid from the urethra; the Skene’s glands produce this fluid. Sexual responses in the female tend to be more prolonged. Detumescence of the female organs is similar to detumescence of the penis. Orgasm in women seems to be learnt, whereas in men, it is a reflex action; female orgasm is not essential for pregnancy.
Systemic effects occur in both males and females: heart rate and blood pressure increase, accompanied by peripheral vasodilatation. This is followed by the resolution phase.
The sperm (Fig. 6.1) develop in the seminiferous tubules of the testes (see Chapter 2). At about 40–50 μm long, of which only approximately 5 μm is the head, the sperm cell is one of the smallest human cells. It retains fertilizing ability for about 2–5 days once deposited in the female reproductive tract. The genetic material of the male gamete is carried in the head of the sperm at the tip of which is located the acrosome, a vesicle containing digestive enzymes. The mid-piece is located between the head and the tail and is packed with mitochondria which generate the ATP required for movement. The tail of the mature sperm has a whip-like action which generates the propulsion for the sperm to swim about 30 cm/h. Abnormalities in human sperm morphology (such as having no tail, two tails or a coiled tail, or no head, two heads or a small head) are very common. Table 6.2 summarizes these and their possible causes. Abnormal motility or morphology of sperm is associated with infertility, causing problems with both fertilization and implantation. As well as the paternal haploid genome (23 paternal chromosomes), the sperm contributes the signal which initiates metabolic activation of the oocyte and also the centriole which causes the microtubules to form the mitotic spindle.
(Reproduced with permission from Brooker, 1998.)
Female choice and male-to-male competition prior to copulation are examples of sexual selection (Lewis et al., 2008). However, it seems that in many species, females typically mate with more than one male at a time when they could conceive. Sperm competition is the competition between sperm from two or more males to fertilize an ovum. There are various mechanisms involved such as sperm number and sperm length, removal of rival sperm, switching off female receptivity to subsequent males and plugging the female reproductive tract. It was proposed that morphologically abnormal human sperm, rather than being due to errors in production, have a role to play in fertilization (Baker and Bellis, 1988). This highly controversial theory suggested that there are two types of sperm: ‘egg-getters’ and ‘blockers’. The morphologically abnormal sperm may be adapted to non-fertilizing roles, particularly preventing passage and successful fertilization by a competitor’s sperm. However, the theory has been contested because the metabolic cost of producing non-fertilizing sperm is high and sperm competition is probably better achieved by better swimming and fertilizing ability rather than by producing sperm to compete in other ways (Lewis et al., 2008). Comparative physiology predicts that mammalian species that have a large testis:body weight ratio are more likely to benefit from a promiscuous mating system in which sperm competition operates (because larger testes produce more sperm); by these criteria, men were not designed to be promiscuous (Short, 1997).
The ovum (i.e. the oocyte surrounded by the corona radiata) is expelled from the mature follicle of the ovary (see Chapter 4) and picked up by the fimbria of the uterine tube. The oocyte is the largest human cell, approximately 120–150 μm in diameter, and is therefore just visible to the naked eye. In comparison, follicular cells are typical-sized human cells of about 10 μm diameter. A rim of these follicular cells known as cumulus cells or the corona radiata surrounds the released oocyte. During follicular growth, the oocyte accumulates RNA and protein and numerous large mitochondria, which provide the needs of the dividing zygote. At birth, meiotic division has been suspended (see Chapter 7).
The lifespan of the human oocyte is thought to be about 6–24 h. Sperm have a viability of up to about 5 days in oestrogenized cervical mucus, so sperm in the reproductive tract up to 5 days before ovulation have a chance of fertilizing an oocyte. This means that the ‘fertile window’, when conception is possible, is about 4–5 days before ovulation. Conception on the day after ovulation has never been reported (Stanford et al., 2002). Sperm concentrations fall with increasing frequency of intercourse but not to an extent that daily intercourse reduces benefit. Sperm counts are maximal after about 5 days of abstinence. Thus, daily intercourse during the fertile window optimizes conception because each day of intercourse increases the probability of pregnancy. The best outcome might be achieved by couples abstaining from intercourse for about 5 days prior to the fertile window and then aiming for daily intercourse.
In meiotic division, the cells have their genetic complement reduced from 46 to 23 chromosomes. Each normal sperm will have 22 autosomes and either an X or a Y sex chromosome. If the oocyte is fertilized by a sperm bearing an X chromosome (a gynosperm), the zygote will be female, and if it has a Y chromosome (an androsperm), the offspring will be male (see Chapter 5). Theoretically, there will be equal numbers of gynosperm and androsperm. However, the sex ratio is not constant. The number of male babies born exceeds the number of female babies all over the world and, since the actual number of male conceptions is proportionately higher, male embryos have a higher failure rate (Bromwich, 1991).
It was observed that the incidence of male babies was markedly higher in cultures where menstruating women were considered unclean and had a ritual cleansing period (niddah) before resuming sexual relations (Harlap, 1979). It was therefore suggested that sperm bearing X and Y chromosomes swim at different rates. If there is a longer period between menstruation and the first intercourse, sperm deposition is less likely to occur much before ovulation, so a recently ejaculated sperm will achieve fertilization. It is hypothesized that androsperm, which are slightly smaller than gynosperm and have rounder heads, can swim faster.
There are a number of methods that are proposed to alter the sex chromosome ratio in sperm and allow sex selection. The difference in mass of gynosperm and androsperm in some species is much more marked than it is in humans. Bulls’ sperm, for instance, can be effectively separated by differential centrifugation. The bottom fraction in the tube will be enriched with the heavier gynosperm, which can be used to impregnate cows to increase the female dairy proportion of the herd. Separation of human androsperm and gynosperm seems more difficult but is practised albeit with questionable success rates. Some environmental factors may influence the ratio of X- and Y-bearing sperm (James, 2008). Although a number of practices may have no scientific foundation, an increased number of female babies are born as the father ages, infants with blood group O are more likely to be male and environmental pollution seems to increase the number of female babies born. Sex selection is practised but usually not at the time of fertilization. In some parts of the world, selective abortion or infanticide is reported. In the West, sex selection is evident in that couples who have both a male and a female child are less likely to have further children.
Case study 6.1 looks at the question of sex determination.
Molly, who has four healthy daughters, is expecting her fifth child. She jokes with the midwife that she knows that this will be a girl as well. The midwife asks her why and Molly informs her that her husband works on an oil rig and is always home for 10 days and then away for 10 days. She laughingly states that every time she has conceived she has always started her period on the day her husband returns home.
• What factors in Molly and her husband’s life may influence the sex of their children?
• How unusual is it to have five children all of the same sex?
In the days leading up to ovulation, the epithelial cells lining the uterine tubes become more ciliated and smooth muscle activity of the tubes increases. At ovulation, the fimbriae of the uterine tube move closer to the ovary and rhythmically stroke its surface. These sweeping movements, together with the currents generated by the moving cilia, facilitate the capture of the ovum released at ovulation. Ovum capture is remarkably efficient. Some women have only one functional ovary and one functional uterine tube; even if the ovary and uterine tube are from opposite sides, pregnancy can still occur. The oocyte is transported towards the uterus by movements generated by peristaltic contractions of the uterine tube aided by the beating movement of the cilia. The oocyte has no inherent motility but is washed along by tubal fluid secreted by the epithelial cells and serum transudate. It takes about 3 or 4 days to reach the uterus. Initially, the movement through the ampulla, where fertilization is most likely to occur, is slow, but the zygote travels faster through the isthmus into the uterus. The junction between the uterine tube and the uterus relaxes under the influence of progesterone and allows the oocyte through. If the oocyte has not been fertilized, it degenerates and is phagocytosed.
Spermiogenesis (see Chapter 2) occurs in the seminiferous tubules but, although the sperm are morphologically mature, they are not fully motile. The sperm develop swimming ability during a maturation phase of 4–12 days in the epididymis. At intercourse, about 200–300 million sperm are released in about 3 mL of seminal fluid (Box 6.1), which is deposited in the vagina. Repeated ejaculation normally results in a fall in sperm concentration, but the proportion of motile sperm decreases in men who are infertile, suggesting that impaired transport through the male genital tract affects motility (Matilsky et al., 1993). The sperm coagulate in the vagina, which appears to facilitate their retention and to buffer them against the normally unfavourable acidic environment (pH ≈ 4–5) of the vagina. The pH of the vagina is increased by the buffers in the seminal fluid favouring sperm motility and access to the cervix. The coagulum dissolves in about 20–60 min.
Box 6.1
• 40–250 million sperm
• Prostatic fluid (30%): citric acid, acid phosphatase, magnesium and zinc ions
• Seminal fluid (60%): fructose (energy source for sperm), alkaline
• pH 7.0–8.3
• Volume: 2–6 mL
Between days 9 and 16 of the menstrual cycle, during the fertile period of the few days preceding and including ovulation, the watery composition of cervical mucus facilitates passage of sperm (see Table 4.1, p. 83). The cervical mucus interacts with the sperm and provides protection and nourishment. It also acts as a reservoir and may filter out sperm with abnormal morphology and motility (Suarez and Pacey, 2006). Most sperm (99%) do not enter the uterus. A few hundred sperm reach the uterine tubes within a few hours of coitus; this first wave of rapid transport probably depends on rhythmic muscular contractions of the female reproductive tract. Some sperm appear to be stored in a reservoir (or at least remain in a functional and fertile state associated with the isthmic epithelium) within the uterine tubes for up to 24 h; they are activated by ovulation. Muscular activity of the female genital tract does not seem to be essential for fertilization; some sperm will be stored in cervical crypts and then travel in a relatively slow second wave through the cervical mucus, reaching the uterine tube a few days after ejaculation. Chemoattractants, released from the ovum and the surrounding cumulus cells and temperature changes, guide the sperm to the cumulus cell oocyte complex (Sun et al., 2005).
Ejaculated sperm are unable to fertilize an oocyte immediately; in order to fertilize the oocyte, ejaculated sperm undergo capacitation, interact with the zona pellucida and undergo the acrosome reaction. In vitro, there may be a delay of several hours before unprepared sperm can fertilize an oocyte. However, in vivo (and in sperm reclaimed from the uterus), the action of female enzymes and the oestrogen-stimulated high salt concentration of the uterine secretions speed up the preparation of the sperm. These biochemical and functional changes undergone by the sperm in the uterus and uterine tubes are known as capacitation. The changes include removal of adherent seminal plasma proteins from the sperm, remodelling of the sperm plasma membrane, including changes in cholesterol and phospholipid content, influx of extracellular calcium, increase in cAMP, phosphorylation of proteins and a decrease in intracellular pH (Wassarman, 2009). The modifications alter ion channels in the membrane allowing a transmembrane flux of ions, which direct protein phosphorylation, thereby initiating hyperactivation of the sperm. Sperm metabolism changes from oxidative to glycolytic. The hyperactivated sperm tail movements change to become whiplash-like so the sperm thrusts vigorously forward, moving from the sperm storage reserve in the isthmus of the uterine tube to the ampulla (Suarez, 2008). The accentuated lateral head movements generate a boring action, which aids access through the cumulus cells and ZP to the oocyte. The tail is also involved in sperm movement within the oocyte (Van Blerkom et al., 1995).
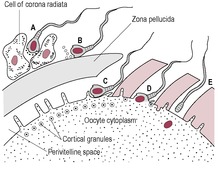
The ovulated oocyte is surrounded by two layers which need to be penetrated by the sperm in order for it to reach the oocyte membrane and to acquire fusibility. The first barrier preventing access of the sperm to the oocyte is the outer layer of cumulus cells, the corona radiata, embedded in an intercellular matrix of carbohydrates, protein and hyaluronic acid. Hyaluronidase, released from the sperm acrosome, breaks down the hyaluronic acid matrix between the follicular cells so sperm can pass through to the zona pellucida. The hyperactive swimming movements of the sperm aid penetration of the corona radiata. The gradual release of sperm from the reservoir of cervical mucus and their activation close to the oocyte means that the time limit of fertility is extended.
The oocyte is surrounded by the zona pellucida, which is about 14–15 μm thick. It is an extracellular matrix composed of sulphated glycoproteins that were produced by the growing oocyte. It is permeable to some viruses, immunoglobulins and enzymes. Before ovulation, cytoplasmic processes from the corona radiata cells penetrate the zona pellucida, allowing communication to, and nourishment of, the oocyte via gap junctions. When these are withdrawn in response to the luteinizing hormone (LH) surge, they may leave gaps in the zona pellucida that offer easier access for sperm penetration, thus facilitating fertilization (Familiari et al., 1992). The zona pellucida acts as a barrier that allows only species-specific sperm–egg interaction.
The human zona pellucida is composed of four glycoproteins ZP1, ZP2, ZP3 and ZP4 (Gupta et al., 2009). ZP2 and ZP3 are implicated as sperm receptors; ZP3 and ZP4 are involved in the binding of sperm to the oocyte and induction of the acrosome reaction. Penetration through the zona pellucida gives the sperm access to the perivitelline space where interaction with the oocyte membrane can take place. The zona pellucida confers a high degree of species specificity; if it is removed, sperm from a different species are able to fertilize an oocyte, though development will rapidly arrest. This is the basis of the hamster zona-free ovum (HZFO) test or sperm penetration assay (SPA) in which hamster oocytes, with their zona chemically removed, are used to test the ability of human sperm to penetrate an oocyte.
The zona pellucida is antigenic: anti-zona pellucida antibodies may be the cause of some cases of infertility. The composition of the zona pellucida changes with the cortical reaction after fertilization so it prevents polyspermy but allows secretions of the uterine tube to reach the oocyte during the early stages of cell division. The zona pellucida also has a role in preventing the blastocyst from prematurely implanting into the wall of the uterine tube before it reaches the uterus. It is possible that an excessively thick zona pellucida could cause problems with blastocyst hatching and subsequent implantation.
Interaction of sperm with the zona pellucida seems to occur in several stages (Ikawa et al., 2010). At first, the capacitated sperm loosely and reversibly adhere to the surface of the zona pellucida. Then, the sperm become strongly and irreversibly bound to the zona pellucida. Many sperm bind to the oocyte zona pellucida but usually only a few sperm penetrate into the perivitelline space and only one will fuse with the oocyte plasma membrane.
After binding to the zona pellucida, the sperm undergo the acrosome reaction (Fig. 6.2). The sperm binding to the ZP receptor is a carbohydrate-mediated event which initiates a signal transduction cascade and raised intracellular calcium concentration. Carbohydrate-binding proteins on the sperm surface recognize glycoproteins on the ZP. This molecular interaction is associated with inhibition of both the innate and adaptive immune responses (Barroso et al., 2009) which may help to protect the gametes and developing embryo (‘fetoembryonic defence system hypothesis;’Clark et al., 2001). The acrosome reaction can also be triggered by follicular fluid and progesterone (Brucker and Lipford, 1995). The outer acrosome membrane fuses with the covering plasma membrane of the sperm. Small vesicles containing acrosomal enzymes are pinched off and their contents are released. The inner acrosomal membrane is then exposed. A tunnel is digested through the zona pellucida by acrosin, a serine protease that remains bound to the inner acrosomal membrane, and the acrosomal enzymes released from the vesicles. The lurching movements of the sperm propel it forward through the zona pellucida and the perivitelline space so that its head is in contact with the oocyte vitelline (surface) membrane. Penetration through the zona pellucida requires both hyperactivated sperm and lysis of the zona pellucida.
The acrosome reaction triggers changes in the sperm membrane that allow sperm–oocyte binding and then fusion to occur. The acrosome reaction reveals antigens such as Izumo on the sperm which can interact with receptors on the surface of the oocyte (Ikawa et al., 2010). Similar egg cell surface proteins on the oocyte membrane are recognized by the sperm. The surface of the oocyte is covered with microvilli, except in the region overlying the meiotic spindle. Oocyte microvilli surround the head of the sperm preceding fusion and then the sperm plasma membrane is incorporated into the oocyte membrane. Various docking and recognition molecules on both the sperm head and the oocyte are implicated in sperm–oocyte binding and fusion; these include sperm ADAMs (a family of proteins with a disintegrin and metalloprotease domain) and CD9 and other proteins on oocytes (Kaji and Kudo, 2004). In human fertilization, the sperm tail remains motile and is incorporated into the oocyte (Payne et al., 1997). Paternal mitochondria entering the oocyte are selectively degraded (Kaneda et al., 1995). The zygote and resulting embryo have only maternal mitochondria (Gyllensten et al., 1991); the oocyte seems to lose mitochondrial DNA (mtDNA) with increasing maternal age, a factor that may be important in fertility. One technique to deal with this is transfer of the cytoplasm from a younger woman’s oocytes to ‘rescue’ oocytes from older women (Barroso et al., 2009). As well as the male nuclear component, the fertilizing sperm also contribute to the centriole and microtubule-organizing centre, from which the first mitotic spindle will develop (Van Blerkom et al., 1995). Fertilization takes about 10–20 min.
Polyploidy is usually fatal and is often detected in spontaneously aborted fetuses (Gardner and Evans, 2006). Most human triploidy is due to polyspermic fertilization where two sperm fertilize an ovum. The number of sperm reaching the newly ovulated egg is partly regulated by sperm transit from reservoirs in the female reproductive tract. Oocytes from most mammals develop mechanisms during growth and development to block polyspermy, the entry of more than one sperm. Following fertilization by one sperm, polyspermy is prevented by a sequence of events which modifies the structure of the plasma membrane and/or the zona pellucida. The relative importance to the plasma membrane versus the zona pellucida block to polyspermy varies with species. In most mammalian oocytes, the zona pellucida is thought to provide the most important block to polyspermy. The number of supernumerary sperm found in the perivitelline space between the ZP and the plasma membrane is usually 1–10 in humans, suggesting that the membrane block occurs almost simultaneously with the ZP block. Two events are involved: the cortical reaction and modification of the zona pellucida by enzymes released from the cortical granules (the ‘zona reaction’). Failure of either of these steps results in polyspermy. Polyspermy results in non-diploid zygotes which are usually not viable but, in some cases, can develop into gestational trophoblastic neoplasias and tumours such as the benign hydatidiform mole or the malignant choriocarcinoma (Hauzman and Papp, 2008). The incidence of non-diploid zygotes increases with alcohol, drug use, anaesthesia and fertilization of ‘aged’ oocytes (i.e. aged in terms of hours after ovulation). Intracytoplasmic sperm injection (ICSI) does not trigger the membrane block to polyspermy; this is not important in the clinical scenario, as ICSI-fertilized eggs are not going to be exposed to additional sperm, but it does indicate that ICSI is not fully equivalent to IVF or in vivo fertilization.
The secretion of cortical granules into the perivitelline space is triggered by a rise in intracellular calcium ion (Ca2+) concentration. In experimental species used to study fertilization, such as sea urchin eggs, two mechanisms have evolved to reduce the occurrence of polyspermy. Prior to the ubiquitous zona reaction, sperm binding rapidly triggers an influx of sodium ions, leading to depolarization of the oocyte membrane which transiently blocks binding of additional sperm. This is known as the ‘fast block’ to polyspermy. There is no evidence for membrane depolarization being involved in the fast block to polyspermy in mammals.
The initial calcium increase triggers calcium release from the intracellular stores, which promotes fusion of the cortical granules with the oocyte membrane (Hoodbhoy and Talbot, 1994). The calcium signal starts from the site of fusion and moves as an oscillating wave through the oocyte, sequentially activating about 4000 cortical granules (Fig. 6.3). The trigger for this calcium-induced calcium rise seems to be a soluble cytosolic factor released from the fertilizing sperm. This sperm factor is probably a novel phospholipase C, PLC-ζ (PLC-zeta) which diffuses into the oocyte cytoplasm following fusion of the sperm and oocyte plasma membranes, initiating the calcium release that leads to the oscillations of calcium (Swann et al., 2004).
These calcium oscillations may last for several hours (Palermo et al., 1997) and act as the signal for the oocyte to activate and begin development. During the ICSI procedure used in IVF (see below), it is common practice to immobilize the sperm by mechanical damage to the sperm tail just before injecting it into the egg (Yanagida et al., 2001). The extensive disruption of the sperm membrane wrought by this damage may help the sperm factor to be released, as there is a more rapid onset of calcium oscillations after ICSI.
Egg activation is triggered by the rise in intracellular calcium concentration; the earliest indicators are the resumption of meiosis and secretion of cortical granules. The rise in calcium subsequently initiates the first division of the embryo (Jones, 2007).
The contents of the cortical granules (enzymes, such as proteases and peroxidase, and polysaccharides) are released into the perivitelline space and diffuse through the zona pellucida to digest the ZP3 sperm receptors. The zona pellucida loses its ability to bind to sperm and to induce the acrosome reaction. The changed texture of the zona pellucida is described as ‘zona hardening’. This reaction is known as the zona reaction. The composition of the oocyte plasma membrane is also altered.
Prior to fertilization, the chromosomes of the oocyte had been arrested in metaphase of the second nuclear division (see Fig. 7.11, p 151). It has been suggested that growth of the oocyte requires a diploid number of chromosomes. Meiotic arrest in the diplotene stage of metaphase means that both maternal and paternal alleles can be expressed during oocyte maturation. Observation of IVF has shown that the human meiotic spindle is unstable and very sensitive to external influences.
Get Clinical Tree app for offline access