Chapter 16. Endocrine system IV. Hormones and metabolism
The adrenal glands
LEARNING OBJECTIVES
At the end of this chapter, the reader should be able to:
• list the different classes of adrenocortical hormones
• give examples of synthetic glucocorticoids
• describe the physiological actions of cortisol
• describe the phases of the survival response to stress and the role played by cortisol
• explain why the physiological actions of cortisol are not the same as the pharmacological actions of clinically used glucocorticoids
• explain the consequences and dangers associated with prolonged use of glucocorticoids and explain the consequences of aldosterone excess
• discuss the dangers associated with the sudden cessation of long-term glucocorticoid therapy
• list the important uses of the glucocorticoids, including replacement therapy in Addison’s disease
The two adrenal glands are situated at the upper pole of the kidneys. They consist of an outer layer or cortex and a central portion or medulla (Fig. 16.1). These two parts of the adrenal gland produce hormones of very different composition and function and they will therefore be considered separately.
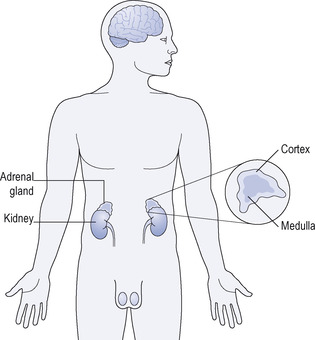 |
| Figure 16.1 Anatomical location and structure of the adrenal gland. |
The cortex
A number of hormones are produced by the adrenal cortex. They also belong to the class of chemical substances known as steroids. Three main groups may be identified:
• mineralocorticoid hormones
• adrenal sex hormones
• glucocorticoid hormones (also called corticosteroids or simply ‘steroids’).
Mineralocorticoid hormones are concerned with salt (sodium) and water control; the most important is aldosterone. Aldosterone increases reabsorption of sodium by the kidney, thus raising the amount of sodium in the body, which in turn causes water retention. The main trigger to the release of aldosterone is the renin mechanism (see p. 74) and its main function is to ensure that the volume of fluid in the circulation and tissue spaces is kept constant.
Excess of aldosterone gives rise to hypertension and sometimes oedema. Very rarely, aldosterone-producing tumours arise in the adrenal gland, causing Conn’s syndrome, which is characterized by hypertension and low plasma potassium with muscle weakness. Many of the synthetic steroids, such as prednisolone and dexamethasone, which are widely used to treat inflammatory conditions, will in higher doses cross-react with the aldosterone receptor and cause oedema. Aldosterone is not available for clinical use as a drug.
Loss of the adrenals is potentially lethal because of the lack of the salt-retaining hormone. The adrenals may need to be removed in patients with breast cancer, to remove any source of sex hormones, and these patients need to be given salt replacement therapy.
Adrenal sex hormones are only secreted in small amounts and are of comparatively little importance as sex hormones when compared with the role of the gonadal sex hormones in sexual reproduction. Both male and female sex hormones are secreted. Excessive secretion of male sex hormones such as androstenedione and testosterone, for example from adrenal tumours in women, leads to virilism.
Glucocorticoid hormones (corticosteroids, steroids) are concerned with metabolism of carbohydrate, fat and protein and will also modify the response of the body to injury. The chief glucocorticoid released from the adrenal is cortisol. Another, minor corticosteroid, namely cortisone, is released as well.
Glucocorticoid hormones
Control of cortisol release
Cortisol release is controlled by corticotrophin (adrenocorticotrophic hormone; ACTH), which is produced by the anterior pituitary (see p. 171). The release of ACTH is in turn stimulated by corticotrophin-releasing hormone (CRH), a hypothalamic releasing hormone. The mechanism is such that when the amount of cortisol in the blood increases it ‘switches off’ the release of corticotrophin by the pituitary and the release of CRH in the hypothalamus. This is a negative feedback action that prevents large changes in the blood cortisol concentration (see Fig. 13.3).
Classification
In addition to cortisol and cortisone, there are a number of synthetic compounds with similar actions to those of cortisol. The members of the whole group are commonly called the corticosteroids, glucocorticoids or simply the ‘steroids’.
Synthetic glucocorticoids:
• betamethasone
• dexamethasone
• fludrocortisone
• methylprednisolone
• prednisolone
• prednisone
• triamcinolone.
Actions of cortisol and other corticosteroids
These can be considered as:
• physiological actions
• pharmacological actions.
Physiological actions of cortiso
Cortisol is the major naturally occurring glucocorticoid hormone in humans. In the blood, cortisol is carried mostly bound to a specific protein, corticosteroid-binding globulin (CBG). CBG also binds progesterone. Only the free, unbound fraction of cortisol is available to the tissues; CBG thus acts as a buffer, preventing excess amounts of cortisol from gaining access to the cells.
It is important to distinguish between the physiological and pharmacological actions of the corticosteroids. These are often confused. The physiological actions are those of the hormone cortisol after it is released from the gland in order to perform its normal role in the body. Cortisol:
• raises blood glucose
• promotes survival responses to stress
• controls ACTH and CRH release.
Effects on blood glucose
Cortisol has both anabolic and catabolic actions. In the liver it stimulates the production of several key enzymes involved in gluconeogenesis, i.e. production of newly synthesized glucose. This is an anabolic action. In fat and muscle, however, cortisol stimulates the breakdown of these tissues to mobilize energy. This is a catabolic action that also results in an increase in glucose synthesis.
Survival response to stress
Cortisol plays a critical role in the body’s response to stress, and if one understands the stress response it makes the actions of cortisol much easier to understand. The body’s response to stress is called the general adaptation syndrome (GAS) and has three components: (1) an alarm reaction, followed by (2) resistance to the stress, which is followed by (3) exhaustion. The alarm reaction involves the release of adrenaline and noradrenaline from the adrenal medulla (see below) and the release of noradrenaline from sympathetic nerve terminals. At the same time, the corticosteroids are released from the adrenal cortex and these permit the released catecholamines to exert their full effects. The resistance phase involves the prolonged effects of cortisol in stimulating gluconeogenesis in the liver and the breakdown of energy stores from fat and muscle. If the stress is prolonged, this will induce the signs of the third phase of exhaustion (which is also seen after prolonged use of corticosteroids in therapy); i.e.
Get Clinical Tree app for offline access



