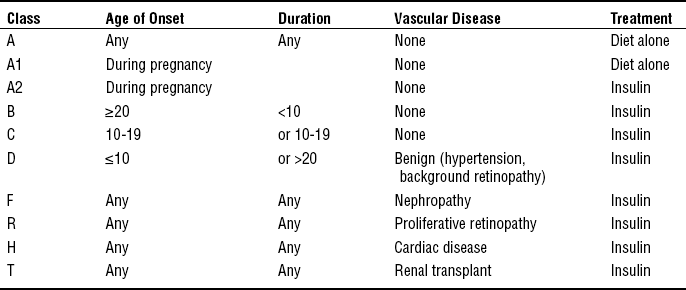
CHAPTER 22 1 Describe maternal and fetal complications associated with endocrine and metabolic disorders in pregnancy. 2 Recognize alterations in pregnancy associated with various endocrine and metabolic disorders. 3 Identify signs and symptoms associated with endocrine and metabolic disorders diagnosed prior to conception or during pregnancy that require specific nursing interventions and care for the childbearing woman and her fetus. 4 Evaluate significant clinical signs and symptoms characterized by various endocrine and metabolic disorders during the childbearing period. 5 Plan assessments and interventions essential in caring for the childbearing woman with an endocrine or metabolic disorder. 6 Implement specific educational content and strategies to empower the childbearing woman with an endocrine or metabolic disorder to knowledgeably participate in her plan of care throughout the childbearing experience. 1. Chronic, systemic endocrine disorder of insulin production or of the body’s response to insulin 2. Caused by absent or inadequate insulin secretion or increased cellular resistance to insulin, resulting in its impaired utilization. 3. Characterized by an abnormal metabolism of carbohydrates, proteins, fats, and electrolytes, resulting in hyperglycemia and other systemic metabolic disturbances 4. Might be associated with severe neurologic, cardiovascular, ocular, renal, and microvascular complications 5. Classified according to cause rather than treatment 6. Complicates more than 200,000 pregnancies per year in the United States (American Diabetes Association [ADA], 2009a) 1. Type 1 diabetes mellitus (formerly insulin-dependent diabetes mellitus [IDDM]) a. Cause is an absolute deficiency of insulin secretion by the pancreatic beta cells b. Might occur by an autoimmune process involved in beta-cell destruction, genetic defects, and disease of the pancreas, endocrinopathies, and use of certain drugs c. Usually appears before the age of 30 years d. Has an abrupt onset of symptoms requiring prompt medical treatment e. Approximately 5% of those diagnosed with diabetes have type 1 diabetes. 2. Type 2 diabetes mellitus (formerly non–insulin-dependent diabetes mellitus [NIDDM]) a. Cause is a combination of resistance to insulin action and an inadequate compensatory insulin secretory response b. Type 2 diabetes is diagnosed primarily in adults older than 30 years of age, but is now seen more frequently in children. Is often associated with obesity and sedentary lifestyle. c. Disease is typically symptom-free for many years, with slow onset and gradual progression of symptoms. d. Type 2 diabetes increases with age, accounting for approximately 95% of all diagnosed cases of diabetes. e. Disease is managed with diet and exercise: The use of oral hypoglycemic medications and/or insulin might also be indicated when hyperglycemia persists. Almost all need insulin for optimum control during pregnancy. 3. Gestational diabetes mellitus (GDM) a. Defined as any degree of glucose intolerance with onset or first recognition during pregnancy (Metzger et al, 2007); further categorized into A1 (controlled with diet and exercise) and A2 (controlled with diet, exercise and requiring the addition of oral meds and/or insulin) b. Estimated to occur in approximately 4% of pregnancies; however, prevalence might range from 1% to 14%, depending on the population studied and diagnostic test used (ADA, 2009a). Accounts for approximately 90% of all diabetes in pregnancy. c. Women diagnosed with GDM are at increased risk for developing diabetes (DM2) later in life. d. Symptoms are generally mild and not life-threatening in the pregnant woman. e. Maternal hyperglycemia is associated with increased fetal morbidity secondary to fetal hyperinsulinemia, which potentiates fetal size (large for gestational age [LGA] or macrosomia greater than 4500 g); therefore, maintenance of normal glucose levels is required for optimal perinatal outcome (Table 22-1). TABLE 22-1 Blood Glucose Values in Pregnancy ∗These values are demonstrated in women with neither diabetes nor carbohydrate intolerance during pregnancy. Sources: Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., Coustan D.R., Hadden D.R., McCance D.R., Hod M., McIntyre H.D., Oats J.J., Persson B., Rogers M.S., Sacks D.A., (2008). HAPO Study Cooperative Research Group: Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine, 358(19), 1991-2002;Combs, C.A., Gavin, L.A., Gunderson, E., Main, E.K., & Kitzmiller, J.L. (1992). Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care, 15, 1251-1257. 4. Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) D Maternal metabolism and pathophysiology in pregnancy 1. Changes in carbohydrate, protein, and fat metabolism in normal pregnancy are profound, mediated in part by the developing fetus and production of placental hormones. 2. First half of pregnancy is considered an anabolic phase (protein and fat storage). a. In pregnant women without diabetes, this is associated with increased estrogen and progesterone secretion, leading to pancreatic beta-cell hyperplasia and hyperinsulinemia. b. Increased insulin production leads to an increased tissue response to insulin and increased uptake and storage of glycogen and fat in the liver and other tissues. 3. Second half of pregnancy is characterized by a catabolic phase (protein and fat breakdown) with increased insulin resistance due to the production of placental hormones (prolactin, human chorionic somatomammotropin [HCS]), cortisol, and growth hormones, which are diabetogenic and act as insulin antagonists; in women who cannot meet the increasing demands for insulin production, this leads to altered carbohydrate metabolism and progressive hyperglycemia; characteristics of this catabolic phase include: a. Increased production of HCS b. Elevated levels of estrogen, progesterone, blood triglycerides, free fatty acids, and serum cortisol c. Tendency for decreased glycogenesis and increased lipolysis, gluconeogenesis, and ketone production; maternal lipolysis provides maternal fuel needs while sparing glucose for fetal use, creating a starvation-like state in the mother (accelerated starvation of pregnancy) 4. The developing fetus continuously removes glucose and amino acids from the maternal circulation. a. Glucose and amino acids are readily transported across the placenta to the developing fetus; insulin is not easily transported. b. Maternal hyperglycemia leads to fetal beta-cell hyperplasia and fetal hyperinsulinemia. 5. The constant transport of maternal glucose levels across the placenta leads to lowered blood glucose levels (hypoglycemia) and explains the lower fasting blood glucose levels observed during normal pregnancy. Note: Fasting blood glucose levels decline 10% to 20% in the first trimester when fetal demands for glucose are low. E Primary goals in the treatment of diabetes and pregnancy Pregestational Diabetes (Type 1 and Type 2 Diabetes) a. Type 1 diabetes is primarily a chronic autoimmune disorder resulting from the destruction of the pancreatic beta-cells, which usually leads to absolute insulin deficiency. b. Type 2 diabetes arises because of insulin resistance, sometimes combined with relative insulin deficiency. a. Incidence of pregestational diabetes is approximately 1% of all pregnancies and affects 10,000 to 14,000 women annually (American College of Obstetricians and Gynecologists [ACOG], 2005). b. Pregestational diabetes accounts for 10% of all diabetic pregnancies. a. Pregnant women with pregestational diabetes might be categorized prognostically according to the classic system of White, with some minor modifications (Table 22-2). b. The quality of metabolic regulation (diabetic control) throughout pregnancy and the presence or absence of serious complications of diabetes, especially nephropathy, hypertension, and heart disease, account for most of the risks associated with diabetes in pregnancy rather than the genetic characteristics of the maternal diabetes. c. Observe for complications associated with diabetes: ketoacidosis, preeclampsia, and pyelonephritis. (1) Diabetic ketoacidosis (DKA) affects about 5% to 10% of diabetic pregnancies (ACOG, 2005); associated with: (b) Hyperemesis gravidarum contributing to dehydration (c) Tocolytic therapy (beta-sympathomimetic agents) (2) Risk of preeclampsia increases with diabetes; complicates 15% to 20% of diabetic pregnancies versus 5% of nondiabetic pregnancies (ACOG, 2005) (3) Maternal infections occur more frequently in diabetic pregnancies than in nondiabetic pregnancies. a. Preconceptual assessments for preexisting diabetics (1) Classification of diabetes in pregnancy (2) Blood glucose control, HbA1c (glycosylated hemoglobin), and frequency of self-blood glucose monitoring (SBGM) (3) Presence of vascular complications and current vascular status; evaluation of renal, retinal, neural, and cardiac status is recommended if duration of diabetes is longer than 5 years. (4) Thyroid panel (type 1 diabetic women only) (5) Neuropathy, retinopathy, nephropathy and CVD treatment if indicated (6) Adequacy of current diet and plans for dietary adjustments in pregnancy (a) Recommended total calorie intake is 30 kcal/kg prepregnant weight of nonobese individuals given as three meals and three snacks (ACOG, 2005). For obese women with a body mass index (BMI) greater than 30, a calorie restriction to approximately 24 kcal/kg actual weight per day (ACOG, 2005) (b) Recommended dietary composition is 40% to 45% carbohydrate, 12% to 20% protein, and 35% to 40% unsaturated fat (Reece & Homko, 2008). (7) Medications should be evaluated. Current insulin regimen might need to be adjusted to attain euglycemia. Note: Women with type 2 diabetes on oral hypoglycemic agents might need to be controlled on insulin prior to conception (contributing to an increase in weight prior to pregnancy); oral agents (glyburide, metformin) in pregnancy may be considered if compliance with insulin regimen is questionable (Langer, Yogev, Xenakis, & Rosenn, 2005; Rowan, Hague, Gao, Battin, & Moore, 2008). (8) Understanding of self-care responsibilities and comprehensive collaborative management of diabetes in pregnancy to promote optimal perinatal outcomes (9) Current lifestyle and related health habits (1) Adequacy of dietary intake; pattern and composition of intake (a) Frequency and method of testing (b) Pattern and recorded results of SBGM (c) Ability to adjust insulin requirements based on changing pattern of blood glucose levels (3) Insulin administration and intensified insulin therapies [i] Tight control in type 1 diabetes is recommended. The documented methods to achieve tight control include multiple (three or more) daily injections or treatment with an insulin pump (Diabetes Control and Complications Trial [DCCT], 1993). [ii] Multiple injections of regular or lispro (rapid-acting) insulin before meals. Intermediate-acting insulin administered with the evening meal or at bedtime. Lispro has a more rapid onset, an earlier peak, and a shorter duration than regular insulin (ACOG, 2005) and has been shown to be more effective in achieving desired glucose levels and reducing the risk of fetal macrosomia (Perkins, Dunn, & Jagasia, 2007). (b) Continuous subcutaneous insulin infusion using an insulin pump with administration of basal rate and bolus doses (c) Dosage adjustments according to changing insulin requirements during pregnancy to maintain euglycemia; typically, insulin requirements increase by two to three times beginning at approximately 18 weeks, peaking at 36 weeks’ gestation. (d) Human forms of insulin recommended; less likely to result in insulin antigenicity (4) Episodes of maternal hypoglycemia and hyperglycemia (a) Might experience increase in episodes of hypoglycemia; signs of hypoglycemia might be altered and not as readily perceived in pregnancy because the release of normal counter-regulatory hormones can be suppressed (b) Assessment of ketones in the urine is recommended: (5) Urinalysis (UA) and urine culture (UC) are usually obtained each trimester, or if symptoms are present. (6) Evaluation of fetal status [i] Pregnancy dating (estimation of gestational age) [ii] Fetal growth and development; assess for: [iii] Assessment for congenital anomalies: increased risk of neural tube defects and congenital heart disease (b) Maternal serum alpha-fetoprotein (MS-AFP) (e) Maternal assessment of fetal activity and fetal movement counts (f) Amniocentesis for lecithin/sphingomyelin (L/S) ratio and phospholipid phosphatidylglycerol (PG) to assess fetal lung maturity and optimize timing of delivery; indicated if induction is planned before 39 weeks’ gestation (g) Doppler studies using Doppler umbilical and uterine artery velocimetry to assess pregnancies at risk for placental vascular disease; might be particularly helpful in the early detection of fetal growth restriction in women with diabetes and vasculopathy. a. Maternal effects and complications (1) Altered insulin requirements (2) Metabolic disturbances related to hyperemesis, nausea and vomiting of pregnancy, and diabetogenic effects of pregnancy (a) Increased risk of hypoglycemia, especially in first trimester (b) Increased risk of ketoacidosis, especially in second trimester (3) Increased risk of maternal infection related to hyperglycemia (4) Progression and possible acceleration of vascular disease secondary to alterations in diabetic control, including retinopathy, nephropathy, and neuropathy (5) Hydramnios; related to fetal anomalies and fetal hyperglycemia (6) Preeclampsia or gestational hypertension (7) Increased maternal mortality and morbidity, associated with the following: b. Fetal effects and complications (1) Increased incidence of congenital malformations and anomalies, including cardiac, skeletal, neurologic, genitourinary, and gastrointestinal; related to maternal hyperglycemia during organogenesis (first 6 to 8 weeks of pregnancy) (a) The incidence of anomalies can be correlated with HgbA1c levels in the mother. (b) It is suggested that levels be kept below 7% to reduce the incidence of anomalies (Slocum, 2007). (a) Large fetal size; related to fetal hyperinsulinemia (increased risk in mothers without vascular disease; White’s classes A to C (see Table 22-2) [i] Unlike other LGA neonates, the organs of the infant of a diabetic mother (IDM) are affected by the macrosomia (organomegaly), and body fat is increased (ACOG, 2005). [ii] A cesarean section might be warranted if the fetus is estimated to weigh more than 4500 g, to avoid traumatic injury from a vaginal birth (ACOG, 2005). (b) IUGR; related to maternal vasculopathy and decreased placental perfusion (increased risk in mothers with vascular disease; White’s classes D to T (see Table 22-2) (see Chapter 17 for further discussion of risks associated with size and age) (3) Fetal asphyxia; related to fetal hyperglycemia and fetal hyperinsulinemia (4) Birth trauma; related to fetal macrosomia and shoulder dystocia (5) Stillbirth, especially after 36 weeks’ gestation in pregnancies complicated by: c. Neonatal effects and complications (1) Prematurity; related to preterm birth associated with maternal complications (2) Respiratory distress syndrome; related to delayed fetal lung maturity and preterm birth (a) Excess insulin produced by the pancreas of the fetus results in delayed surfactant production, probably by interfering with the lung’s ability to use phospholipids by blocking receptor sites. [i] This delay in surfactant production is found primarily in classes A to C of diabetic amniotic fluid. [ii] Diabetic infants with vascular disease seldom develop respiratory distress syndrome (RDS); chronic stress of poor intrauterine perfusion leads to increased production of steroids, which accelerates lung maturation (Ricci, 2007). (b) To avoid iatrogenic RDS, it is suggested that the usual parameters of lung maturity be adjusted for IDMs. [i] An amniotic fluid L/S ratio of two or greater does not always ensure that lung maturity has been achieved (Ricci, 2007). [ii] The presence of PG is reassuring (see Chapter 17 for a complete discussion of respiratory distress). (3) Metabolic and hematologic disturbances; related to maternal hyperglycemia [i] Glucose molecules readily cross the placenta, but insulin does not readily cross. [ii] The fetus responds by producing large quantities of insulin, leading to hyperinsulinemia. [iii] When the umbilical cord is cut after delivery, the supply of glucose rapidly diminishes, yet the level of insulin remains constant, leading to neonatal hypoglycemia. [iv] Glucose levels should be monitored frequently in the newborn (as per institutional protocol); levels should be greater than 40 mg/dL. If less, offer breast milk or formula; if necessary, intravenous (IV) glucose infusion. [i] Defined as serum calcium less than 7 mg/dL [ii] Is usually manifested in first 2 to 3 days of life [iii] Might occur as result of birth injury or decreased magnesium level, which suppresses parathyroid hormone production, thus decreasing calcium levels (Ricci, 2007) (d) Polycythemia and hyperbilirubinemia [i] Polycythemia is due to the decreased ability of HbA1c in the mother’s blood to release oxygen; subsequent breakdown of increased red blood cells (RBCs) predisposes to hyperbilirubinemia. [ii] Might result from fetal hypoxia, increase in fetal erythropoietin, sequestered blood from birth injuries, and/or impairment of hepatic function by neonatal hypoglycemia that interferes with bilirubin conjugation (see Chapter 17 for a complete discussion of hyperbilirubinemia) (4) Cardiomyopathy and anomalies; related to maternal hyperglycemia (see the previous discussion and Chapter 17 for more information about congenital anomalies) 6. Psychosocial considerations a. Adaptation to presence of chronic illness b. Presence and adequacy of support systems: partner, family, significant others c. Adequacy of coping responses associated with diagnosis of high-risk pregnancy d. Presence of psychosocial problems such as depression, anxiety, and eating disorders e. Financial concerns related to need for more intensive monitoring of pregnancy f. Planned or unplanned pregnancy g. Family’s response to pregnancy h. Feelings regarding high-risk status of pregnancy i. Availability of specialized health care team for management of pregnancy 1. Altered metabolism of carbohydrates, proteins, fats, and electrolytes (1) Assess caloric intake and dietary pattern using 24-hour recall; review importance of regularity of meals when taking insulin. (2) Encourage monitoring blood glucose levels and recording results of testing at least four to seven times daily (before and after meals and at bedtime). Note: Abnormal glucose results are most frequently caused by: (3) Assist with regulation of insulin dosage according to changing physiologic needs and blood glucose levels throughout pregnancy. (a) Might switch to human forms of insulin (b) Intensify insulin regimen with multiple injections three or four (or more) times daily. (4) Encourage urine testing for ketones to identify starvation ketosis or developing ketoacidosis: (b) For blood glucose levels greater than 200 mg/dL (d) When glucose control is altered. Note: Persistent ketonuria might indicate the need for an additional snack or change in insulin regimen. (5) Review signs and symptoms for maternal hypoglycemia, which might be altered during pregnancy; and the prevention and management of hypoglycemic episodes (patients should be instructed to have a source of fast-acting carbohydrate with them at all times, such as six to eight LifeSavers, 120 mL [4 ounces] of fruit juice, or 2 tablespoons of raisins). (1) Appropriate amounts of carbohydrate (CHO), proteins, and unsaturated fats are consumed to maintain blood sugar levels within individualized goals. (2) Blood glucose levels remain within individualized goals determined for optimal maternal and fetal outcome. (3) Insulin dosages are regulated according to changing physiologic needs and maternal blood glucose levels throughout pregnancy. (4) Urine is tested for ketones in the morning, when blood glucose levels are 200 mg/dL or higher, during maternal illness, and when blood glucose control is altered. (5) Signs and symptoms of maternal hypoglycemia and ketoacidosis are recognized promptly and managed appropriately during pregnancy. 2. Anxiety due to high-risk pregnancy status (b) Presence of visual disturbances (c) Signs and symptoms of preeclampsia and urinary tract infections (UTIs) (2) Prompt identification of alterations in clinical assessments and referral for appropriate medical and obstetric management (3) Review potential effects of diabetes on pregnancy to promote understanding of risks and ways to control or minimize them. (1) Alterations in blood pressure, presence of visual disturbances, and signs and symptoms of preeclampsia and UTIs are promptly assessed. (2) Appropriate referrals for medical and obstetric management of clinical alterations in pregnancy are obtained to minimize potential maternal, fetal, and neonatal complications. (3) Effects of diabetes on pregnancy outcome and risk reduction strategies are reviewed. 3. Feelings of powerlessness related to fetal outcome (1) Discuss strategies for maintenance of optimal glycemic control during pregnancy. (2) Provide information about tests and procedures for fetal assessment and surveillance. (3) Encourage active participation in decision making and planning for medical and obstetric care throughout pregnancy. (4) Discuss feelings about pregnancy and self-monitoring practices for management of diabetes during pregnancy. (1) Patient maintains optimal blood glucose control during pregnancy. (2) Patient receives information about tests and procedures for fetal assessment. (3) Patient actively participates in decision making and planning for medical and obstetric care throughout pregnancy. (4) Patient expresses her feelings about her pregnancy and self-monitoring practices for management of diabetes during pregnancy. 4. Plan of diabetes self-care and obstetric management of diabetes during pregnancy (1) Discuss the rationale for blood glucose control and importance of euglycemia before conception and during pregnancy. (2) Review self-care practices. (a) Blood glucose monitoring and frequency of testing (b) Insulin administration; adjustment of insulin dosages based on blood glucose levels (c) Regular daily mild exercise program (3) Refer for dietary counseling to ensure optimal diet for glycemic control and fetal growth and development. (4) Discuss plan of care for obstetric management and fetal surveillance. (1) Patient can verbalize rationale for blood glucose control and importance of euglycemia before conception and during pregnancy. (2) Patient demonstrates proper techniques and frequency for blood glucose monitoring and insulin administration, adjusts insulin dosages based on blood glucose determinations, and modifies dietary intake during pregnancy. (3) Patient receives dietary counseling to ensure optimal diet for glycemic control and fetal growth and development. (4) Patient can verbalize a recommended plan of care for obstetric management and fetal surveillance. 5. Elevated serum glucose levels, changes in circulation (1) Monitor maternal glycemia. (2) Assess fetal well-being, including results of NST, AFP, BPP, Doppler studies, and ultrasound testing. (3) Encourage maternal assessment of fetal movement using daily fetal movement counts. (1) Patient demonstrates dietary management, blood glucose monitoring, and insulin dosage adjustments to maintain euglycemia. (2) Fetal status and well-being are monitored via NST, BPP, Doppler studies, and ultrasound testing. (3) Patient participates in assessment of fetal well-being using daily movement counts and records, and reports changes in the pattern of fetal activity. 6. Demands of recommended diabetes and obstetric care during pregnancy (1) Assess maternal support systems and the presence and involvement of significant others in assisting the patient with self-care behaviors and practices. (2) Assess alterations in maternal work or employment status and potential economic effect of pregnancy, including financial concerns and expenses. (3) Encourage active participation of significant others in prenatal care and testing. (1) Patient describes support systems and the presence and involvement of significant others in the performance of self-care behaviors and practices during pregnancy. (2) Patient expresses financial concerns related to alterations in maternal work or employment status during pregnancy. (3) Patient’s significant others will actively participate in prenatal care and testing. Pregestational Diabetes Mellitus 1. Discussion of potential maternal and fetal risks associated with diabetes and pregnancy, effects of diabetes on pregnancy, and pregnancy on diabetes 2. Discussion of financial expenses and other demands related to the increased surveillance of maternal and fetal status during pregnancy 3. Discussion of rationale for interdisciplinary team approach and role of each team member in the management of diabetes and pregnancy 4. Discussion of rationale for optimal blood glucose control before conception to ensure optimal timing of conception and early diagnosis of pregnancy. Note: Research has demonstrated that near-normal blood glucose levels at the time of conception and in the early weeks of gestation might significantly reduce the increased incidence of congenital anomalies associated with infants of mothers with diabetes (Slocum, 2007). 5. Review of self-care practices and self-monitoring expectations during pregnancy, including diet, intensification of insulin regimen, and multidisciplinary plan for medical and obstetric management 1. Reinforcement of multidisciplinary plan for medical and obstetric management during pregnancy 2. Ongoing assessment of blood glucose levels and adjustment of insulin requirements to ensure euglycemia 3. Discuss minimization and prompt recognition of potential maternal and fetal complications associated with diabetes and pregnancy 4. Exercise is encouraged 30 to 60 minutes per day to increase insulin sensitivity. Monitor blood glucose before and after exercise. If glucose is less than 100 mg/dL, consume carbohydrate to prevent hypoglycemia (ADA, 2009b). 1. A precipitous decrease in insulin requirements in the immediate postpartum period is related to delivery of placenta and cessation of contra-insulin hormones associated with pregnancy; usually persists for at least 72 hours after birth. 2. Breastfeeding is encouraged in women with diabetes. a. Improves glucose metabolism and promotes high-density lipoprotein (HDL) cholesterol b. Might be associated with decreased insulin requirements (up to 27%) c. Necessitates increased calories and continued dietary modifications to ensure adequate nutrition during lactation and milk production d. Might experience increased incidence of mastitis, sore nipples secondary to candidiasis, and hypoglycemic episodes. Note: Maternal hypoglycemia is most likely to occur 1 hour after breastfeeding, and women with preexisting diabetes should be encouraged to eat a small snack just before breastfeeding. e. Hypoglycemia decreases milk production and might lead to problems with establishing milk supply and maintaining lactation. 3. Discuss birth control and preconception care for next pregnancy. 1. Carbohydrate intolerance of variable severity with onset or first recognition during pregnancy 2. Patient might need diet, exercise, and/or insulin for optimum control. 3. Diabetes mellitus might persist after pregnancy. Screening should occur 6 to 12 weeks’s postpartum. 4. Glucose intolerance might have antedated the pregnancy. 5. Some studies suggest oral meds may be used in lieu of insulin (Langer et al, 2005; Rowan et al, 2008). 1. History (preconceptual risk factors associated with GDM) a. Previous delivery of an LGA or macrosomic (>4500 g) infant b. Previous infant with congenital anomaly c. Previous unexplained intrauterine fetal demise (IUFD) or neonatal death d. History of GDM in previous pregnancy e. History of hydramnios in prior pregnancy f. Poor reproductive history (i.e., history of preterm birth or recurrent spontaneous abortions) g. Family history of diabetes (i.e., parent or sibling with diabetes) i. BMI greater than 29 (maternal obesity) (ADA, 2009a) k. Ethnic background: African American, Asian, Pacific Islander, Hispanic, and Native American 2. Physical findings and associated risk factors in current pregnancy (1) Development of hydramnios, suspected large fetal size, or increased fundal height relative to dating of pregnancy (2) Persistent glycosuria on two successive prenatal visits (4) Urinary frequency after first trimester (5) Recurrent monilial infections (6) Reported feelings or behaviors of excessive thirst or hunger (1) Increased fetal size; associated with operative delivery, birth trauma, and shoulder dystocia (5) Neonatal hyperbilirubinemia (6) Respiratory distress syndrome (7) Infants of mothers with fasting and postprandial hyperglycemia are at greatest risk for intrauterine death or neonatal mortality. (8) Overall perinatal mortality has been reported to be 6.4% when GDM is untreated; studies suggest that there is no increase in perinatal mortality when GDM is managed appropriately and maternal glucose levels are normal. 3. Psychosocial considerations—adaptation to diagnosis and management of GDM (see previous section on Pregestational Diabetes, Psychosocial Considerations) (1) The American College of Obstetricians and Gynecologists (ACOG) recommends universal screening (ACOG, 2001); however, published data indicate that universal screening is not cost effective. Recommendation by ADA (2009a) is for selective screening for GDM of pregnant women with one or more of the following criteria: (b) Obesity (BMI of 29 or higher) (c) Family history of type 2 diabetes (first-degree relative) (d) Ethnic group with a high prevalence of type 2 diabetes (e) History of abnormal glucose tolerance (f) History of poor obstetric outcome (f) Diagnosis of polycystic ovary syndrome (PCOS) (a) Women meeting the criteria should undergo a glucose challenge test between the 24th and 28th week of gestation; an earlier screen should be performed on those women with identified risk factors. (b) Remain seated, no smoking, administer 50 g of oral glucose, given without regard to time of day or interval since the last meal. (c) Measure venous plasma glucose 1 hour later; level should be less than 140 mg/dL; any value equal to or greater than 140 mg/dL requires a full 3-hour diagnostic oral glucose tolerance test (OGTT). A glucose threshold of 140 identifies approximately 80% of GDM, and using a threshold value of 130 results in about 10% more abnormal screens. Either threshold is acceptable (ADA, 2009a).
Endocrine and Metabolic Disorders
DIABETES MELLITUS
Ideal∗
Goal
Fasting blood glucose
60-89 mg/dL
<90 mg/dL
1 hour postprandial
100-129 mg/dL
<140 mg/dL
Mean blood glucose
87 mg/dL
<100 mg/dL
Hemoglobin A1c
2%-5%
<7%
CLINICAL PRACTICE
HEALTH EDUCATION
GESTATIONAL DIABETES MELLITUS
CLINICAL PRACTICE
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Endocrine and Metabolic Disorders
Get Clinical Tree app for offline access