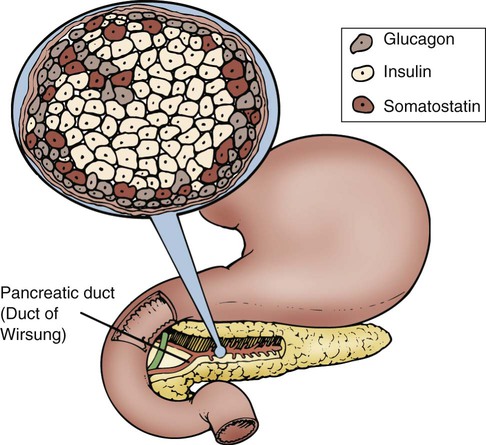
Maintaining dynamic equilibrium among the various cells, tissues, organs, and systems of the human body is a highly complex and specialized process. Two systems regulate these critical relationships: the nervous system and the endocrine system. The nervous system communicates by nerve impulses that control skeletal muscle, smooth muscle tissue, and cardiac muscle tissue. The endocrine system controls and communicates by distributing potent hormones throughout the body. Figure 31-1 lists the endocrine glands and their hormones, target tissues, and actions. When stimulated, the endocrine glands secrete hormones into surrounding body fluids. In the circulation, these hormones travel to specific target tissues, where they exert a pronounced effect. Receptors found on or within these specialized target tissue cells are equipped with molecules that recognize the hormone and bind it to the cell, producing a specific response. Specialized exocrine cells within the pancreas secrete digestive enzymes into a duct that is 3 mm wide and transverses the length of the pancreas. The pancreatic duct joins with the cystic duct, carrying bile from the liver and gallbladder, before it empties into the duodenum at the major duodenal papilla. This common ductal exit for the two organs raises the danger of a gallstone lodging at the duodenal papilla and blocking the outflow of pancreatic enzymes. Pancreas exocrine anatomy is illustrated in Figure 28-9 in Chapter 28, and pancreatic digestive juices are discussed as part of the physiology of the gastrointestinal (GI) system. Most pancreatic tissue is devoted to production of exocrine digestive juices. In the pancreas, clusters of cells that appear to form tiny islands among the exocrine cells accomplish the pancreatic endocrine functions. These clusters are known as the islets of Langerhans and are composed of four distinct cell types: alpha, beta, delta, and PP. The locations of the cells that produce these hormones are shown in Figure 31-2. Alpha cells secrete glucagon, beta cells secrete insulin, delta cells secrete somatostatin, and PP cells secrete pancreatic polypeptide hormone. Glucagon, insulin, somatostatin, and polypeptide hormones are released into the surrounding capillaries to empty into the portal vein, where they are distributed to target cells in the liver. The hormones then travel into general circulation to reach other target cells. Insulin is a potent anabolic hormone produced by the beta cells of the pancreas. Elevated levels of blood glucose stimulate insulin production. Insulin is the only hormone produced in the body that directly lowers blood glucose levels. Insulin is responsible for the storage of carbohydrate, protein, and fat nutrients. Insulin also augments the transport of potassium into the cells, decreases the mobilization of fats, and stimulates protein synthesis (Table 31-1). Box 31-1 defines the terms commonly used when discussing glucose and insulin balance. The major stimulant for insulin secretion is an elevation of serum glucose. The greater the rise in blood glucose, the more insulin the normal pancreas produces. Other hormones inhibit the release of insulin (Table 31-2). TABLE 31-1 SNS, Sympathetic nervous system; ↑, increases; ↓, decreases. TABLE 31-2 AGENTS THAT PROMOTE OR INHIBIT INSULIN RELEASE Glucose is admitted to the skeletal, cardiac, and adipose cells for use as energy in the presence of effective insulin facilitated by the glucose transporter 4 (GLUT4), as described below. The movement of glucose from the circulation into the intracellular compartment reduces the concentration of glucose in the bloodstream and helps preserve the blood’s osmolality. Simultaneously, glucose is available to the cell as its main energy source. Excess glucose in the form of glycogen is stored in the hepatic and muscle cells for use as fuel at a later time. In skeletal muscle, 90% of the glucose in the cell is converted to glycogen for longer-term storage.1 Liver glycogen contributes up to 10% of total liver weight when fully replete.1 Adequate, effective insulin levels affect fat metabolism. Dyslipidemias are strongly associated with type 2 diabetes. Type 2 diabetes is characterized by overproduction of large, triglyceride-rich, very-low-density lipoproteins (VLDLs).2 Disorders of carbohydrate and fat metabolism are also associated with metabolic syndrome, a precursor to diabetes and cardiovascular disease.3 Glucagon, synthesized by alpha cells in the pancreas, has the opposite effect of insulin. Glucagon is released during hypoglycemia to induce hepatic glucose output. Because glucagon counter-regulates insulin levels and raises blood glucose levels, it is a potent gluconeogenic hormone. By means of gluconeogenesis, glucagon can form glucose from noncarbohydrate sources such as fat and protein when required. Glucagon release from the pancreas is stimulated by low blood glucose levels, starvation, exercise, or stimulation of the sympathetic nervous system (SNS), as listed in Box 31-2.4,5 Glucagon release protects the brain from the consequences of hypoglycemia.5 To meet short-term energy requirements, glucagon stimulates the release of glycogen stores from liver and muscle cells. Through a process called glycogenolysis, the glycogen stored in the liver is converted into a glucose form that can be used by the cells.5 For long-term energy needs, glucagon stimulates glucose release through the more complex process of gluconeogenesis. In gluconeogenesis, fat and protein nutrients are rapidly broken down into end products that are then changed into glucose.5 A normal blood glucose level is maintained in the healthy body by the insulin-to-glucagon ratio. When the blood glucose level is high, insulin is released, and glucagon is inhibited. When blood glucose levels are low, glucagon rather than insulin is released to raise the blood glucose level. The brain has a very limited supply of glucose, and glucagon release is essential to protect the brain from the effects of hypoglycemia.6 Somatostatin is a hormone that is produced in the pancreatic delta cells. Somatostatin decreases glucagon secretion, and in high quantities, it decreases insulin release (see Table 31-1). Hyperglycemia stimulates the activity of the delta cells. It is theorized that the release of insulin enables somatostatin to control beta cell activity. Somatostatin may be involved in the regulation of the postprandial influx of glucose into cells. Human cells take up glucose by means of facultative glucose-transport proteins known by the term glucose transporter (GLUT). These proteins are specialized by tissue distribution and function as described in Table 31-3.7 At the cellular level, glucose crosses the cell plasma membrane through aqueous pores formed by GLUT transporters. At this time, 14 GLUTs have been identified.8 The GLUT number indicates the order in which the molecular sequence and GLUT tissue locations were identified.8 The functions of a few key GLUTs are described in more detail below. TABLE 31-3
Endocrine Anatomy and Physiology
Pancreas
Anatomy
Exocrine Cells
Physiology
Insulin
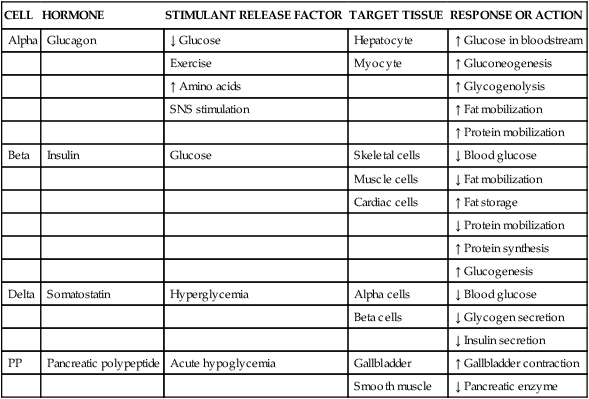
CELL
HORMONE
STIMULANT RELEASE FACTOR
TARGET TISSUE
RESPONSE OR ACTION
Alpha
Glucagon
↓ Glucose
Hepatocyte
↑ Glucose in bloodstream
Exercise
Myocyte
↑ Gluconeogenesis
↑ Amino acids
↑ Glycogenolysis
SNS stimulation
↑ Fat mobilization
↑ Protein mobilization
Beta
Insulin
Glucose
Skeletal cells
↓ Blood glucose
Muscle cells
↓ Fat mobilization
Cardiac cells
↑ Fat storage
↓ Protein mobilization
↑ Protein synthesis
↑ Glucogenesis
Delta
Somatostatin
Hyperglycemia
Alpha cells
↓ Blood glucose
Beta cells
↓ Glycogen secretion
↓ Insulin secretion
PP
Pancreatic polypeptide
Acute hypoglycemia
Gallbladder
↑ Gallbladder contraction
Smooth muscle
↓ Pancreatic enzyme
INSULIN RELEASE (MAJOR STIMULANT: HIGH BLOOD GLUCOSE)
INSULIN INHIBITION (MAJOR INHIBITOR: LOW BLOOD GLUCOSE)
Hormones
Glucagon
Somatostatin
Corticotropic hormone
Norepinephrine
Thyrotropin
Epinephrine
Somatotropin
Glucocorticoids
Incretins
Medications
Beta-adrenergic stimulators
Beta-adrenergic blocking agents
Sulfonylurea
Diazoxide
Theophylline
Phenytoin
Acetylcholine
Thiazide or sulfonamide diuretics
Blood Glucose
Carbohydrate Anabolism.
Fat Anabolism.
Glucagon
Somatostatin
Glucose Transporters
GLUCOSE TRANSPORTER (GLUT)
ANATOMIC LOCATIONS
FUNCTION
GLUT1
Erythrocytes, endothelial cells of brain
Basal glucose uptake
Transport across blood–brain barrier
GLUT2
Pancreatic beta bells, liver, kidney, small intestine
High-capacity, low-affinity GLUT
Can transport fructose
GLUT3
Brain cells, nerve cells
Transports glucose into neural tissue
GLUT4
Striated muscle and adipose tissue
Insulin-regulated transport in muscle and fat
GLUT5
Intestine, kidney, testis
Transports fructose
GLUT6
Spleen, leukocytes, brain
GLUT7
Small intestine, colon, testis
Transports fructose
GLUT8
Testis, brain, muscle, adipocytes
Fuel supply of mature spermatozoa
GLUT9
Liver, kidney
GLUT10
Liver, pancreas
Muscle-specific fructose transporter
GLUT11
Heart, muscle
GLUT12
Heart, prostate, mammary gland
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine Anatomy and Physiology
Get Clinical Tree app for offline access