Week 2
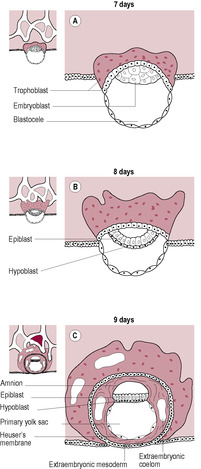
Fig. 9.1
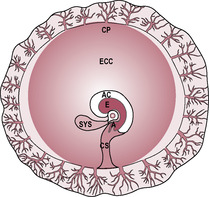
Fig. 9.2
Week 3
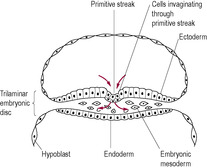
Fig. 9.4
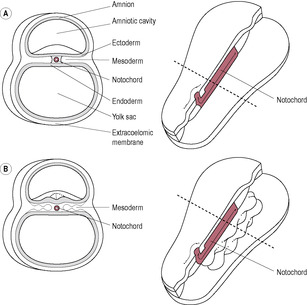
Fig. 9.6
Week 1: fertilization to produce zygote
Week 2: inner cell mass forms bilaminar embryonic disc of hypoblast and epiblast
Week 3: gastrulation
Weeks 4–8: organogenesis
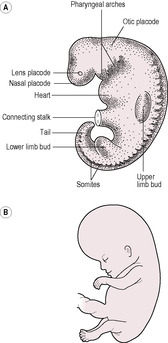
Fig. 9.7
Folding
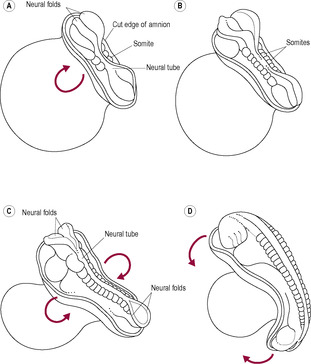
Fig. 9.8
The organization of the basic body plan
4th week
5th week
6th week
7th week
8th week
Ninth week to birth: fetal period
9–12 weeks
13–16 weeks
17–20 weeks
21–25 weeks
26–29 weeks
30–34 weeks
35–38 weeks
Development of organ systems
The central nervous system

Embryo development and fetal growth
Zara is 5 feet and 2 in. tall (155 cm) and, prior to pregnancy, wore size 8 clothes; she wears size 3 (European size 35.5) shoes. Her husband, James, is 6 feet tall (180 cm) and has quite a broad, muscular frame and has size 11 feet (European size 46). Zara has frequently discussed with her midwife her concerns that she will have a big baby and problems in labour because James is tall and has a large frame.
• How can the midwife reassure Zara that the baby’s growth is unlikely to be disproportionate to her size and what physiological measurements and observations can be made to support this?
• If Zara was found to be carrying a large baby how would the midwife be able to recognize this and what could be the possible explanations for this?
• How would having a large baby affect the plan of care for Zara; what are the potential concerns and how could they be mediated?
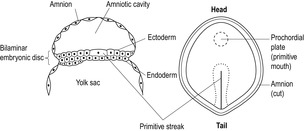
By the end of the first week, the blastocyst has entered the uterine cavity, hatched out of the zona pellucida and started the process of implantation into the endometrial wall. Two types of cells are evident: the outer trophoblast (see Chapter 6) and the inner cell mass (or embryoblast). The inner cell mass gives rise to tissues of the embryo and also contributes towards some of the extraembryonic membranes. The undifferentiated cells from the blastocyst are called stem cells and have some potential therapeutic uses (see Box 9.1). At about day 7 after fertilization, the cells of the inner cell mass start to proliferate and differentiate rapidly. The inner cell mass becomes flattened into a bilaminar embryonic disc with the cells forming two distinct layers (Fig. 9.1). The cells adjacent to the blastocyst cavity appear distinctly cuboidal. These form the hypoblast or primary endoderm layer, which gives rise to the future gut and its derivatives. The upper layer of cells is formed of columnar epiblast cells, which will differentiate into the ectodermal layer. Some of the epiblast cells spread laterally to form the amnioblasts of the amniotic membrane that encloses the amniotic cavity. Cells from the hypoblast layer migrate forming a membrane called the exocoelomic membrane or Heuser’s membrane which lines the cytotrophoblast so the blastocyst cavity is also enclosed. This is the cavity that will become the primitive (primary) yolk sac. There are two waves of endoderm cell remodelling of the blastocyst cavity, which form initially the primary yolk sac and then, at the beginning of the fifth week, the definitive (secondary) yolk sac (Fig. 9.2). The formation of the definitive yolk sac creates the chorionic cavity and the extraembryonic mesoderm, which gives rise to the vascular structures of the placenta (see Chapter 8). The definitive yolk sac synthesizes several proteins including alpha-fetoprotein (AFP). The bi-laminar disc lies between two fluid-filled cavities: the amniotic cavity on the epiblast (ectoderm) side and the yolk sac cavity on the hypoblast (endoderm) side. (The primitive yolk sac will shrink away from the cytotrophoblast in the fourth week, creating a new cavity called the chorionic cavity or extraembryonic coelom, which fills with fluid and becomes the largest cavity in the developing conceptus.)
Box 9.1
Stem cells are unspecialized cells that have the ability to self-renew and to differentiate into a number of cell types. This ability to produce a variety of differentiated cell types means that stem cells are a potential source of replacement cells that could be used to treat variety of diseases and repair damaged tissues. There are several categories of stem cells defined according to the variety of cells they are able to produce. Totipotent stem cells are able to produce all of the cells present in the fetus and adult together with those in the extraembryonic tissues such as placenta. Pluripotent cells are able to produce any cell in the body, multipotent cells can produce particular types of related cells, for example blood cells, whereas oligopotent (a few types of very similar cells) and unipotent (one cell type) have progressively more restricted lineages.
The zygote and cells from only the first few divisions are termed ‘totipotent’. At the fourth cleavage division after fertilization, when the 8-cell embryo divided to form 16 cells, totipotency is lost. Genetic screening can be carried out at the 8-cell stage by the technique of pre-implantation genetic diagnosis by removing one of the eight cells for analysis. Since all cells at this stage are totipotent, the zygote will develop normally from the seven remaining cells. Also, if the cells are separated at this stage and allowed to implant, identical offspring will develop; this is a type of cloning. Following the loss of totipotency, the embryonic cells differentiate into pluripotent stem cells. These pluripotent cells can form any of the three types of germ cell layer (endoderm, mesoderm or ectoderm) and are therefore capable of becoming any cell in the body but cannot form a unique individual because they cannot form extraembryonic mesoderm and placental tissue.
There are a number of sources of stem cells which are usually classified as being embryonic (pluripotent) or adult (multipotent) stem cells. Embryonic stem cells (ESC) can be harvested from inner cell mass of the blastocyst (the trilaminar embryonic disc). Research using ESC is controversial because human ESC are taken from early embryos which are destroyed. ESC can be obtained from a cloned embryo which would make them genetically compatible with the recipient and therefore avoid the issue of immune rejection. A potentially important and recent advance is to ‘persuade’ differentiated cells to return to the undifferentiated stem cell state by expression of specific genes, a technique referred to as induced pluripotency (iPS). This also offers the advantage that somatic cells from an adult can be converted into stem cells and then be used to treat diseases in the same person, again, avoiding the problem of tissue immune rejection.
Adult stem cells are derived from progenitor cell populations such as bone marrow cells which can be differentiated into liver, kidney, muscle and nerve cells as well as blood cells. Skin dermis cells can be transdifferentiated into many types of tissue including neurons, smooth muscle cells and fat cells. It is also possible that brain cells could be harvested from dead organ donors. A major concern of stem cell use is to control stem cell division to ensure that tissues are regenerated but tumours do not grow.
Most adult stem cells are multipotent but some stem cells from the umbilical cord and cord blood cells are pluripotent. It is possible to reprogram multipotent adult stem cells to become pluripotent. In some countries, parents are asked whether they would like to have their baby’s cord blood cells frozen in case they can be used later in life to treat a disease. The blood is taken from the umbilical vein of the cleaned cord once the baby has been delivered; it is checked for infectious agents, tissue-typed and then stored in liquid nitrogen. Stem cells can be used either autologously (for the person they came from) or as an allogenic treatment, where the donor and recipient are different individuals. The information given to new parents suggests a much wider use of the stem cells harvested from their baby’s umbilical blood but many of these applications are currently at the research stage. To date, stem cells have been used in humans to treat leukaemia and restore eyesight using limbal stem cell therapy. The treatment of type-I diabetes, spinal cord injuries, neurodegenerative and other diseases is an active area of research.
(Reproduced with permission from Fitzgerald and Fitzgerald, 1994.)
(Reproduced with permission from Jauniaux et al., 2003.)
At the end of the second week, a region of endodermal cells starts to thicken and become columnar, forming the prochordal plate (Fig. 9.3). This marks the cranial region (head end) and is the site of the future mouth. The prochordal plate is also important in influencing further development of the cranial region (Box 9.2).
Box 9.2
The zygote and cells of the early blastocyst are termed ‘totipotent’ which means that each cell has the ability to develop into all cells of the organism and form all the types of body tissue. At the fourth division after fertilization, when the eight cells present divide forming 16 cells, totipotency is lost. Genetic screening can be carried out by pre-implantation genetic diagnosis before the fourth division by removing one of the eight cells for analysis; the zygote will develop normally from the seven remaining cells. If the cells are separated and implanted, identical offspring will develop; this is a type of cloning. Following the loss of totipotency, the embryonic cells differentiate into pluripotent stem cells. These pluripotent cells can form any of the three types of germ cell layer: endoderm, mesoderm or ectoderm but cannot form a unique individual because they cannot form extraembryonic mesoderm and placental tissue.
By the second week, according to the embryologist’s ‘rule of twos’, the following have taken place:
• two germ layers have formed: the endoderm and the ectoderm
• two trophoblastic layers have formed: cytotrophoblast and syncytiotrophoblast
• two waves of remodelling have occurred: that of the blastocyst into the primary and then the definitive yolk sac
• two novel cavities have formed: the amniotic cavity and the chorionic cavity
• two layers have formed from the extraembryonic mesoderm.
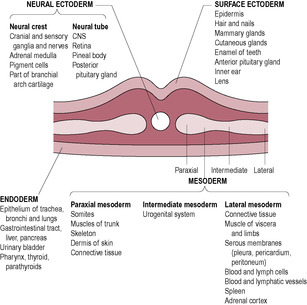
At this stage, when the woman may first realize she is pregnant, embryo development is rapid. A line of epiblast cells, starting from the caudal region (tail end) at the other side from the prochordal plate, undergoes very rapid cell division, forming the primitive streak in the midline (see Fig. 9.3). The cells of the primitive streak form a groove and then invaginate (move inwards) to spread between the epiblast and hypoblast layers. The bilaminar disc is therefore converted into a trilaminar disc consisting of three germ layers (ectoderm, mesoderm and endoderm), which give rise to specific tissues of the body (Fig. 9.4). The middle layer is the mesoderm, from which connective tissue, smooth muscle, the cardiovascular system and blood, the skeleton and the reproductive and endocrine systems develop (Fig. 9.5). The epiblast becomes the ectoderm, which will develop into the epidermis, central and peripheral nervous systems and the retina. Therefore, the ectoderm, which will give rise to the skin, is in contact with the amniotic cavity from very early on in embryonic development. The hypoblast becomes the endoderm, from which epithelial linings and some glandular structures will form. The three germ layers interact, generating signals that induce cellular interactions and cause structural alterations and more complex interactions.
(Reproduced with permission from Fitzgerald and Fitzgerald, 1994.)
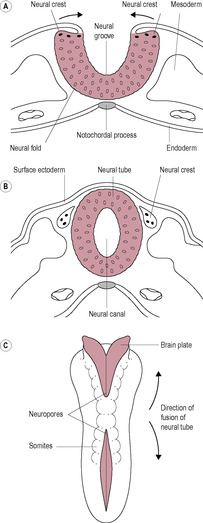
The endodermal prochordal plate is fused to the ectoderm forming the oropharyngeal membrane (future mouth). Below the primitive streak, there is another area of fusion between the ectoderm and endoderm; this is the cloacal membrane (the future anus). Rare birth complications such as imperforate anus may arise from abnormal development of the cloacal membrane. Some mesoderm cells migrate towards the prochordal plate forming a cord of adhesive cells (Fig. 9.6). This is the notochordal process, which develops a lumen forming the notochord canal. The notochord evolves into a cellular rod-like tube, which gives the trilaminar disc a degree of rigidity and defines the central head–tail axis of the embryo. If identical twins are going to develop, there are two parallel notochords. If there are two notochords that cross, conjoined (Siamese) twins will result; on the position where the notochords cross dictates where the twins will be conjoined, for example twins with cephalic joining have a higher cross-over point of their notochords than twins who are joined at the hips (Spitz, 2005). The notochord establishes the development of the axial skeleton (bones of head and spinal cord) and the neural plate, which gives rise to the primitive nervous system. The vertebral column forms around the notochord and the notochord induces neurulation, the formation of the neural tube and early nervous system (see below). During the third week, aggregates of mesoderm on either side of the notochord form pairs of bead-like blocks called somites, which direct the segmented structure of the body and induce the overlying ectoderm to form structures of the nervous system.
(Reproduced with permission from Goodwin, 1997.)
The formation of the primitive streak, the three germ layers, the prochordal plate and the notochord is described as gastrulation. Gastrulation marks the beginning of morphogenesis, the emergence and development of body form and structure. It begins with the appearance of the primitive streak at day 14. The primitive streak defines the time when experimental manipulation of human embryos is legally obliged to stop under the terms of the UK Human Fertilization and Embryology Act of 1990 (amended 2008). Gastrulation is a very sensitive stage of embryogenesis; the cell populations are very vulnerable to teratogenic insult at the beginning of the third week of development (Sadler, 2010). For instance, high levels of alcohol can kill cells in the craniofacial region of the embryonic disc affecting brain and face development. The very rare tumours of the neonate can be a result of remnants of primitive streak proliferating to form sacrococcygeal tumours. During the third week of development, as well as gastrulation, the primitive nervous system and cardiovascular system begin to develop.
Box 9.3 is a summary of the events taking place in weeks 1–3.
Box 9.3
• Cleavage of zygote while travelling in uterine tube
• Cell division without increase in mass to form morula
• Fluid accumulation: hollow blastocyst formed
• ‘Hatching’ out of zona pellucida
• Blastocyst cells differentiate into trophoblast and inner cell mass
• Implantation in decidual wall
• Trophoblast differentiates into dividing cytotrophoblast and invasive syncytiotrophoblast (see Chapter 8)
• Lateral movement of cells from epiblast layer encloses yolk sac, forming the extraembryonic mesoderm
• Prochordal plate (mouth) develops at caudal end
• Day 14: primitive streak develops
• Cells from primitive streak invaginate and migrate between the epiblast and the hypoblast forming the mesoderm
• Trilaminar disc of three germ layers: ectoderm (epiblast), mesoderm and ectoderm (hypoblast)
• Notochord forms, inducing development of the neural plate and giving rise to axis of development
• Somites become evident
• Neurulation begins
During this period of embryonic development, the trilaminar disc folds into a C-shaped cylindrical embryo and all the major structures and organ systems are established. However, apart from the cardiovascular system, few of the systems function. Organogenesis, the development of the organ systems, is a critical period during which the processes are susceptible to external influences that can cause disruption and subsequent serious congenital abnormalities. By the end of the eighth week, the embryo becomes known as the fetus and has a distinct human appearance (Fig. 9.7). Human development can be crudely classified as three types:
• growth: cell division
• morphogenesis: development of form, which involves movement of sheets and masses of cells
• differentiation: maturation of cells forming tissues and organs capable of specialized function.
((A) Reproduced with permission from Fitzgerald and Fitzgerald, 1994.)
Growth is achieved by hyperplasia (cell division) and hypertrophy (increase in cell size). Initially the cells are stem cells which are similar and not differentiated or specialized into any particular cell type. They differentiate into 1 of the 350 different types of cell found in the body in two phases. Before differentiation occurs, there is a stage of determination during which the cell becomes restricted in its capability to develop along different pathways. As the cells differentiate fully, they develop specific morphological and functional characteristics.
Differentiation is often orchestrated by the establishment of a signalling centre or polarizing region in a small bud of undifferentiated cells, as occurs in the development of the vertebrate limb. This process in which cells in one place influence the surrounding cells to develop in a specific way is known as induction. Induction involves the surrounding cells (‘inducers’) to produce cellular signals which have an effect on the responding cells (‘responders’) via cell receptors. Competence is the capacity to respond to the signal from an inducer which requires the responding tissue to be activated by a competence factor (Sadler, 2010). These interactions often involve epithelial cells, which are usually joined together forming tubes or sheets, and mesenchyme cells which are more dispersed. For instance, the epithelial cells forming the lining of the gut interact with the neighbouring mesenchyme cells to form the gut-associated organs such as the pancreas and liver and the epithelial tissue interacts with the limb mesenchyme cells to produce the initial limb buds. Continued signalling or ‘crosstalk’ between the different cell types allows differentiation to progress. The crosstalk occurs by the cells producing growth and differentiation factors which act at a paracrine (involving diffusible factors) or juxtacrine (involving non-diffusible factors) level.
Paracrine factors act by triggering a signalling transduction pathway in which a signalling molecule (or ‘ligand’) interacts with its receptor often conferring enzymatic activity to the receptor so it is able to initiate a cascade of protein phosphorylation changes that terminate with a transcription factor being activated. The transcription factor can then activate or inhibit gene expression. Juxtacrine factor signalling involves proteins on the surface of one cell or in the extracellular matrix interacting with the receptor on the surface of another cell or signals being transmitted from one cell to another via gap junctions. Differentiation can allow some plasticity. Branching morphogenesis is the formation of branched epithelial tubules which is essential to the development of several tissues including the kidneys, lungs, breasts and salivary glands.
One of the cornerstones of embryology is the concept that germ cells of the three layers of the developing embryo migrate to the final destinations (Horwitz and Webb, 2003). The migrating cells are thought to be polarized (have a front and back), to sense chemical signals and ‘home’ towards them by amoeboid movement. In gastrulation, cell migration leads to the formation of the three layers of the trilaminar disc which then migrate to targets where they differentiate. The muscle precursor cells migrate from the somites to their targets in the limbs. Failure of cells to migrate at all or to the correct location is thought to result in abnormalities or to have life-threatening consequences; for instance, congenital defects in brain development leading to mental disorders are ascribed to defects in neuronal migration. Cell migration also occurs in post-natal life and is central to homeostasis such as effective immune responses and repair of injured tissues in the wound healing process. Migration can also occur in pathological processes, including vascular disease and tumour metastasis, where some tumour cells migrate to new sites where they form secondary tumours. However, recent research has questioned whether germ cells do actually migrate at all during ontogeny (Freeman, 2003).
Apoptosis or programmed cell death is another mechanism important in embryonic development. Apoptosis involves the cells effectively autodestructing in a precisely timed manner. Embryogenesis also involves cell recognition and adhesion.
The disc-like arrangement of the germ layers is converted into a recognizable vertebral embryo by folding in the fourth week of development. Folding is due to a differential rate in growth of the different parts of the embryo. The embryo is in a contained space so as it grows, it curves and ridges of tissues form. The embryonic disc grows rapidly particularly in length, because of the growth of the brain and tail, so it has to fold. Although this is a momentous stage of development, relatively little is known about it. The yolk sac does not grow and, as the outer rim of the endoderm is attached to the yolk sac, the embryo becomes convex. Folding occurs at the cephalic (head) and lateral regions on day 22 and at the caudal (tail) end of the embryo on day 23 (Fig. 9.8). The cephalic, lateral and caudal edges of the embryonic disc are brought into apposition and the layers fuse along the midline, which converts the endoderm into the gut tube. Initially the foregut and the hindgut fuse, leaving the midgut open to the yolk sac. The folds cause a constriction between the embryo and yolk sac. The yolk sac gives rise to the primitive gut. The amnion expands, enveloping the connecting stalk and neck of the yolk sac, forming the umbilical cord. Folding is precisely coordinated and is controlled. Failure at this point results in conditions such as omphalos and gastroschisis. The developmental pattern involves synchronized tissue communication and interaction. Adjacent tissues induce changes in the movement and behaviour of neighbouring cells. Signals integrating genetic and environmental influences control cell proliferation, migration and apoptosis (Bard and Weddon, 1996). These signals, which may be diffusible molecules or direct physical contact, direct the expression of particular genes in the responding cells. Although all cells have the same DNA in their nuclei, depending on the signal received, some will express certain genes but not others. So, for instance, a skin cell expresses the genes that control the behaviour of a skin cell because they are switched on by the signals skin cells receive. A liver cell has the same genes as the skin cell but expresses different genes.
(Reproduced with permission from Goodwin, 1997.)
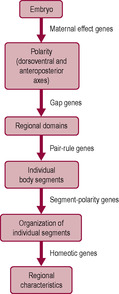
Techniques and concepts used to study molecular genetics (how the genetic code is expressed) in bacteria and Drosophila can be applied to mammalian embryogenesis, including human development. The DNA in the nucleus sets up a basic body plan, which establishes the pattern of the early embryo. The genes that control the basic body plan are the same in very diverse species. A highly conserved region of about 180 base pairs of DNA, known as the homeobox, is found in the genes that regulate the craniocaudal (head–tail) axis of embryonic development in almost all species studied (Murtha et al., 1991). The homeobox encodes a protein domain called the homeodomain which can bind DNA specifically. Homeobox genes encode transcription factors which switch on cascades of other genes, for instance all the ones needed to make a particular body part. Other morphogenic agents, signals and growth factors activate the homeobox genes. Hox genes are a particular subgroup of homeobox genes which are found in a special gene cluster, the HOX cluster. Hox genes determine the patterning of the body axis. They direct the identity of particular body regions, determining where limbs and other body segments will develop in the fetus. Limb abnormalities such as polydactyly may result from abnormal Hox genes. There appears to be a series of three sequential steps in the conversion of the oval trilaminar embryonic disc into the cylindrical configuration with the endoderm on the inside, the ectoderm on the outside and the mesoderm in between (Fig. 9.9) (Carlson, 2008). These steps result in segmentation of the embryo. Gap genes subdivide the embryo into broad regional domains. Pair-rule genes are involved in the formation of individual body segments and segment-polarity genes control the anterior–posterior organization of each segment (De Robertis et al., 1990). As the embryo develops, the segmental plan becomes less evident; remnants can be seen in the arrangement of the backbone and ribs and in the organization of the spinal nerves.
Box 9.4 summarizes the events taking place during weeks 4–8.
Box 9.4
• Neural tube fusing but neuropores open at rostral (anterior) and caudal ends
• Folding produces characteristic C-shaped curved embryo
• Otic pits present (primitive ear)
• Optic vesicles formed
• Upper limb buds appear, then lower limb buds
• Three pairs of brachial arches present
• Beating heart prominent
• Forebrain prominent
• Attenuated tail
• Rudiments of organ systems established
• Rostral neuropore, then caudal neuropore, close
• CRL 4–6 mm
• Rapid brain development and head enlargement (cephalization)
• Facial prominences develop
• Upper limb buds become paddle-shaped
• Lower limb buds are flipper-like
• Mesonephric ridges denote position of mesonephric (interim) kidneys
• CRL 7–9 mm
• Joints of upper limbs differentiate
• Digital rays (fingers) of upper limbs evident
• External ear canal and auricle (pinna) formed
• Retinal pigment formed so eye is obvious
• Head very large, projects over heart prominence
• Reflex responses to touch
• CRL 11–14 mm
• Notches between digital rays partially separate future fingers
• Liver prominent
• Rapidly growing intestines herniate out of small abdominal cavity into umbilical cord
• CRL 16–18 mm
• Digits of hand separated (but still webbed)
• Notches visible between digital rays of feet
• Stubby tail disappears
• Purposeful limb movements occur
• Ossification begins in lower limbs
• Head still disproportionately large (about half of total embryo length)
• Eye lids closing
• Ears are characteristic shape but still low-set
• External genitalia evident (but not distinct enough for sexual identification)
• CRL 27–31 mm
During this period, the body grows rapidly and the tissues and organs differentiate and mature (see below). The head growth rate becomes relatively slower so, by birth, the length of the head is about a quarter of the total length. Growth rate can be used to determine embryonic or fetal age (Box 9.5) and ultrasound examination can be used to examine developmental details (Box 9.6). With expert care, a fetus can be viable and may survive from 22 weeks.
Box 9.5
• Greatest length (GL) is used to measure embryos of about 3 weeks, which are straight
• CRL is sitting height, used to measure older, curved embryos
• Carnegie embryonic staging system uses external characteristics to estimate developmental stage
• Number of somites
• Fetal head measurements, such as biparietal diameter and head circumference
• Abdominal circumference
• Femur length and foot length
Box 9.6
• Estimation of size and age of embryo
• Detection of congenital abnormality
• Evaluation of growth rate
• Investigation of uterine abnormality or ectopic pregnancy
• Guidance for CVS
Box 9.7 is a summary of the changes during the fetal period.
Box 9.7
• Growth in body length and limbs accelerates
• Ears are low-set, eyes are fused
• Primary ossification centres develop in skeleton, notably skull and long bones
• Intestines return to abdominal cavity and body wall fuses
• Erythropoiesis (formation of red blood cells) decreases in liver and begins in spleen
• Urine formation begins
• Fetal swallowing of amniotic fluid
• Rapid growth
• Coordinated limb movements (not felt by mother)
• Active ossification of skeleton
• Slow eye movements
• Ovaries differentiated and contain primordial follicles
• External genitalia recognizable
• Eyes and ears closer to normal positions
• Growth slows down
• Limbs reach mature proportions
• Fetal movements felt by mother (‘quickening’)
• Skin covered with protective layer of vernix caseosa, held in position by lanugo (downy hair)
• Brown fat deposited
• Fetus gains weight
• Skin wrinkled and translucent, appears red–pink
• Rapid eye movements begin
• Blink-startle responses to noise
• Surfactant secretion begins but respiratory system immature
• Fingernails are present
• May be viable if born prematurely
• Lungs capable of breathing air
• Central nervous system can control breathing
• Eyes open
• Toenails visible
• Fat (3.5% body weight) deposited under skin so wrinkles smooth out
• Erythropoiesis moves from spleen to bone marrow
• Pupillary light reflex
• Skin pink and smooth, limbs chubby
• White fat is 8% of body weight
• From 32 weeks, survival is usual
• Firm grasp
• Orientates towards light
• Circumference of head and abdomen are approximately equal
• White fat is about 16% of body weight, 14 g fat gained per day
• Skin appears bluish-pink
• Term fetus is about 3400 g, CRL is about 360 mm
Neurulation is the formation of the neural plate and neural folds and the closure of these folds to form the neural tube, which is the precursor of the brain and spinal cord. The neural tube is completed by the end of the fourth week. The developing notochord induces the overlying ectoderm to thicken forming the neural plate, a raised slipper-like plate of neuroepithelial cells. This will give rise to the central nervous system (brain and spinal cord) and other structures such as the retina. In the middle of the third week, the neural groove appears in the centre of the neural plate (Fig. 9.10). To each side of the groove are neural folds, which enlarge at the cranial end as the start of the developing brain. Marked development of the brain is a characteristic of embryonic development in primates; human brain growth exceeds that of other species, continuing into adulthood. At the end of the third week, the neural folds start to fuse forming the neural tube, which separates from the surface ectoderm. The neural crest cells, which detach from the lateral edges of the neural folds, give rise to the spinal ganglia and ganglia of the autonomic system as well as a number of other cell types (Box 9.8). The paraxial mesoderm, closest to the notochord and developing neural tube, differentiates to form prominent paired blocks of tissue, or somites. The first somites appear from day 20. There are about 30 pairs of somites by day 30 increasing to a total of 44 pairs, but the cranial ones begin differentiation as new somites are added at the caudal end. The somites differentiate into sclerotomes, myotomes and dermatomes, which give rise to the axial skeletal bones, skeletal muscles and the dermis of the skin, respectively. The number of somites indicates the age of the embryo. The limbs carry with them the nerves from the somites from which they developed. The somatic pattern of development is important in understanding referred pain (see Chapter 13).
Box 9.8
Get Clinical Tree app for offline access
• Spinal ganglia
• Ganglia of the autonomic system
• Adrenal medulla
• Thyroid gland
Get Clinical Tree app for offline access