Do not confuse eculizumab with efalizumab or palivizumab, or Soliris with Synagis. (Apo-Enalapril Do not confuse enalapril with Anafranil, Elavil, Eldepryl, or ramipril.
E
eculizumab
Indications/routes/dosage
Paroxysmal nocturnal hemoglobinuria
Atypical hemolytic uremic syndrome
Body Weight
Induction
Maintenance
40 kg and over
900 mg weekly × 4 doses
1,200 mg wk 5, then 1,200 mg q2wks
30–39 kg
600 mg weekly × 2 doses
900 mg wk 3, then 900 mg q2wks
20–29 kg
600 mg weekly × 2 doses
600 mg wk 3, then 600 mg q2wks
10–19 kg
600 mg weekly × 1 dose
300 mg wk 2, then 300 mg q2wks
5–9 kg
300 mg weekly × 1 dose
300 mg wk 2, then 300 mg q3wks
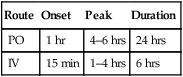
enalapril
![]()
![]() , Epaned, Novo-Enalapril
, Epaned, Novo-Enalapril ![]() , Vasotec)
, Vasotec)
E
Get Clinical Tree app for offline access

 classification
classification classification
classification
 classification
classification classification
classification classification
classification classification
classification classification
classification