Maintenance dosing in adults and pediatric patients over 10 years of age:
2.4 to 3.6 mcg/kg/day given once daily. Doses may be adjusted every 2 weeks according to clinical response, serum drug levels, and toxicity.
Pediatric dose
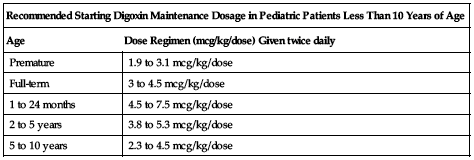
Use 0.1 mg/mL pediatric injection (100 mcg/mL). If using a tuberculin syringe to measure a pediatric dose, do not flush syringe with parenteral solution after contents are injected; may result in an overdose. The recommended starting maintenance dose in pediatric patients less than 10 years of age is listed in the following chart. These recommendations assume the presence of normal renal function.
| Recommended Starting Digoxin Maintenance Dosage in Pediatric Patients Less Than 10 Years of Age | |
| Age | Dose Regimen (mcg/kg/dose) Given twice daily |
| Premature | 1.9 to 3.1 mcg/kg/dose |
| Full-term | 3 to 4.5 mcg/kg/dose |
| 1 to 24 months | 4.5 to 7.5 mcg/kg/dose |
| 2 to 5 years | 3.8 to 5.3 mcg/kg/dose |
| 5 to 10 years | 2.3 to 4.5 mcg/kg/dose |
Dose adjustments
The following two charts provide recommended maintenance doses for specific patient populations based on lean body weight and renal function.
| Digoxin Maintenance Dose (in mcg given once daily) in Adults and Pediatric Patients Over 10 Years of Age Based on Lean Body Weight and Renal Function | ||||||||
| Corrected Creatinine Clearance* | Lean Body Weight (kg)‡ | Number of Days Before Steady State Achieved† | ||||||
| 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||
| 10 mL/min | 64 | 80 | 96 | 112 | 128 | 144 | 160 | 19 |
| 20 mL/min | 72 | 90 | 108 | 126 | 144 | 162 | 180 | 16 |
| 30 mL/min | 80 | 100 | 120 | 140 | 160 | 180 | 200 | 14 |
| 40 mL/min | 88 | 110 | 132 | 154 | 176 | 198 | 220 | 13 |
| 50 mL/min | 96 | 120 | 144 | 168 | 192 | 216 | 240 | 12 |
| 60 mL/min | 104 | 130 | 156 | 182 | 208 | 234 | 260 | 11 |
| 70 mL/min | 112 | 140 | 168 | 196 | 224 | 252 | 280 | 10 |
| 80 mL/min | 120 | 150 | 180 | 210 | 240 | 270 | 300 | 9 |
| 90 mL/min | 128 | 160 | 192 | 224 | 256 | 288 | 320 | 8 |
| 100 mL/min | 136 | 170 | 204 | 238 | 272 | 306 | 340 | 7 |
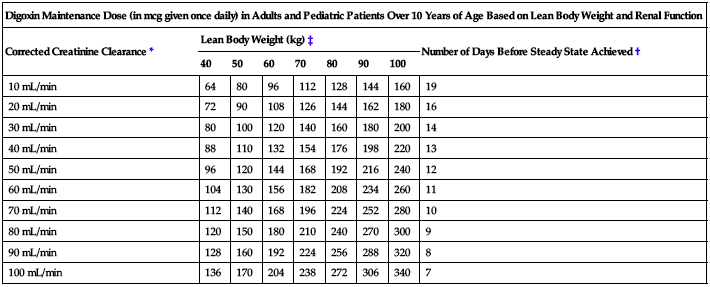
*For adults, CrCl was corrected to a 70-kg body weight or 1.73 M2 body surface area. For pediatric patients, the modified Schwartz equation may be used. The formula is based on height in cm and SCr in mg/dL where k is a constant and CCr is corrected to 1.73 M2 body surface area. During the first year of life, the value of k is 0.33 for preterm infants and 0.45 for term infants. The k is 0.55 for pediatric patients and adolescent girls and 0.7 for adolescent boys: GRF mL/min/1.73 M2 = (k × Height)/SCr
†If no loading dose administered.
■ Alternatively, the maintenance dose for adults and pediatric patients over 10 years of age may be estimated using the following formula:
Total maintenance dose (mcg) = Loading dose (mcg) × % Daily loss ÷ 100
Where % daily loss = 14 + CrCl ÷ 5.
| Digoxin Maintenance Dose* (in mcg given TWICE daily) in Pediatric Patients Under 10 Years of Age Based on Lean Body Weight and Renal Function | ||||||||
| Corrected Creatinine Clearance‡ | Lean Body Weight (kg)† | Number of Days Before Steady State Achieved § | ||||||
| 5 | 10 | 20 | 30 | 40 | 50 | 60 | ||
| 10 mL/min | 8 | 16 | 32 | 48 | 64 | 80 | 96 | 19 |
| 20 mL/min | 9 | 18 | 36 | 54 | 72 | 90 | 108 | 16 |
| 30 mL/min | 10 | 20 | 40 | 60 | 80 | 100 | 120 | 14 |
| 40 mL/min | 11 | 22 | 44 | 66 | 88 | 110 | 132 | 13 |
| 50 mL/min | 12 | 24 | 48 | 72 | 96 | 120 | 144 | 12 |
| 60 mL/min | 13 | 26 | 52 | 78 | 104 | 130 | 156 | 11 |
| 70 mL/min | 14 | 28 | 56 | 84 | 112 | 140 | 168 | 10 |
| 80 mL/min | 15 | 30 | 60 | 90 | 120 | 150 | 180 | 9 |
| 90 mL/min | 16 | 32 | 64 | 96 | 128 | 160 | 192 | 8 |
| 100 mL/min | 17 | 34 | 68 | 102 | 136 | 170 | 204 | 7 |
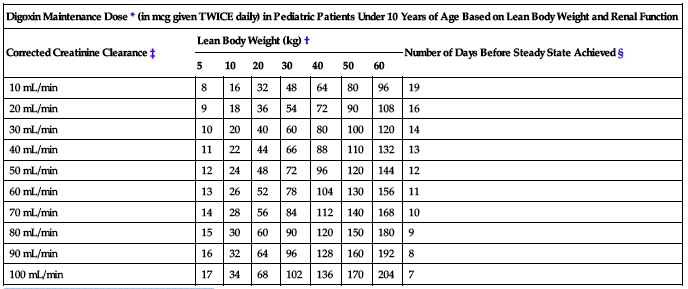
*Recommended doses are to be given twice daily.
†The doses listed assume average body composition.
‡The modified Schwartz equation may be used to estimate creatinine clearance. See preceding chart.
■ Reduce dose in patients whose lean weight is an abnormally small fraction of their total body mass because of obesity or edema. ■ Monitor for S/S of digoxin toxicity and clinical response. Adjust dose based on toxicity, efficacy, and blood levels. ■ Reduce dose in partially digitalized patients, in patients with impaired renal function, and in the elderly. ■ Dose reduction may be required before cardioversion. ■ See Drug/Lab Interactions; adjustments may be required with numerous drugs. ■ Reduced doses may be indicated in advanced heart failure, myocardial infarction, severe carditis, or severe pulmonary disease. ■ Renal clearance diminished in neonates, including premature infants; adjust dose as indicated. ■ See Precautions.
Dilution
Available as a 500 mcg (0.5 mg) in 2 mL injection (250 mcg [0.25 mg] per mL) and as a 100 mcg (0.1 mg) in 1 mL (pediatric) injection. May be given undiluted or each 1 mL may be diluted in 4 mL SWFI, NS, or D5W. Less diluent may cause precipitation. Use diluted solution immediately. Give through Y-tube or three-way stopcock of IV infusion set. See Pediatric Dose.
Storage:
Store unopened vials at CRT protected from light.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer recommends not mixing with other drugs in the same container and not administering simultaneously via the same IV line.
One source suggests the following compatibilities (not recommended by manufacturer):
Additive:
Furosemide (Lasix), lidocaine, ranitidine (Zantac), verapamil.
Y-site:
Anidulafungin (Eraxis), bivalirudin (Angiomax), ceftaroline (Teflaro), ciprofloxacin (Cipro IV), cisatracurium (Nimbex), dexmedetomidine (Precedex), diltiazem (Cardizem), doripenem (Doribax), famotidine (Pepcid IV), fenoldopam (Corlopam), heparin, hetastarch in electrolytes (Hextend), hydrocortisone sodium succinate (Solu-Cortef), insulin (regular), linezolid (Zyvox), meperidine (Demerol), meropenem (Merrem IV), midazolam (Versed), milrinone (Primacor), morphine, nesiritide (Natrecor), potassium chloride (KCl), remifentanil (Ultiva), tacrolimus (Prograf).
Rate of administration
Each single dose over a minimum of 5 minutes. Avoid bolus administration. Rapid administration may cause systemic and coronary arteriolar constriction.
Actions
A cardiac glycoside obtained from Digitalis lanata. Inhibits Na-K ATPase, the “sodium pump” responsible for moving sodium ions out of cells and potassium ions into cells. The cardiologic consequences of this action are an increase in the force and velocity of myocardial systolic contraction (positive inotropic action), a slowing of the heart rate (negative chronotropic effect), decreased conduction velocity through the AV node, and a decrease in the degree of activation of the sympathetic nervous system and renin-angiotensin system (neurohormonal deactivating effect). Increases cardiac output and left ventricular ejection fraction and lowers pulmonary artery pressure, pulmonary capillary wedge pressure, and systemic vascular resistance. Widely distributed throughout the body. Onset of action is 5 to 30 minutes. Time to peak effect is 1 to 4 hours. Half-life is 1.5 to 2 days. Minimal metabolism. Rapidly excreted in urine, primarily as unchanged drug. Crosses the placenta and is secreted in breast milk.
Indications and uses
Treatment of mild to moderate heart failure in adults. ■ To increase myocardial contractility in pediatric patients with heart failure. ■ Control of ventricular response rates in adult patients with chronic atrial fibrillation; see Precautions.
Contraindications
Ventricular fibrillation and known hypersensitivity to digoxin or other digitalis preparations.
Precautions
IV administration is the preferred parenteral route. Used only when oral therapy is not feasible or rapid therapeutic effect is necessary. ■ Calcium channel blockers (e.g., diltiazem [Cardizem], verapamil) or beta blockers (e.g., atenolol [Tenormin], metoprolol [Lopressor]) are generally preferred for rate control in patients with atrial fibrillation; adenosine (Adenocard) is preferred to treat PSVT. ■ Commonly prolongs the PR interval; may cause severe sinus bradycardia or sinoatrial block in patients with pre-existing sinus node disease and may cause advanced or complete heart block in patients with pre-existing incomplete AV block. Consider insertion of a pacemaker before treatment with digoxin in these patients. ■ Use in patients with an accessory AV pathway (Wolff-Parkinson-White syndrome) increases risk of ventricular fibrillation and is not recommended. ■ Avoid use in patients with heart failure associated with preserved left ventricular systolic function (e.g., acute cor pulmonale, amyloid heart disease, constrictive pericarditis, idiopathic hypertrophic subaortic stenosis, restrictive cardiomyopathy). Patients with these conditions may experience a decreased cardiac output. Has been used for ventricular rate control in a subgroup of these patients with atrial fibrillation. ■ Avoid use in patients with myocarditis; may precipitate vasoconstriction. ■ Use with caution in patients with impaired renal function; risk of toxicity increased; see Dose Adjustments. ■ Use caution in patients with electrolyte disorders because potassium or magnesium depletion sensitizes the myocardium to digoxin; toxicity may occur with serum digoxin concentrations below 2 ng/mL. ■ Patients with beriberi heart disease may fail to respond adequately to digoxin if the underlying thiamine deficiency is not treated concomitantly. ■ Use with caution in patients with hypercalcemia. May increase risk of digoxin toxicity. Maintain normocalcemia. ■ Hypocalcemia may nullify effect of digoxin. If calcium levels need to be restored to normal, give calcium slowly and in small amounts. Serious arrhythmias have occurred in digitalized patients receiving calcium. ■ Hypothyroidism may reduce requirements for digoxin. Addressing the underlying condition is suggested in patients with heart failure and/or atrial arrhythmias resulting from hypermetabolic or hyperdynamic states (e.g., hyperthyroidism, hypoxia, or arteriovenous shunt). Atrial arrhythmias associated with hypermetabolic states are particularly resistant to digoxin treatment. ■ Use is not recommended in patients with myocardial infarction. May cause an increase in myocardial oxygen demand and ischemia. ■ Some clinicians suggest reducing or discontinuing the dose of digoxin for 1 to 2 days before an elective cardioversion to avoid the induction of ventricular arrhythmias, but physicians must consider the consequences of increasing the ventricular response if digoxin is decreased or withdrawn. If countershock is necessary (last-resort treatment of life-threatening arrhythmias), begin with low voltage levels and increase gradually to avoid ventricular arrhythmias. ■ See Drug/Lab Interactions.
Monitor:
Monitor for S/S of digoxin toxicity (anorexia, nausea, vomiting, vision changes, cardiac arrhythmias). ■ Low body weight, advanced age, impaired renal function, hypomagnesemia, hypokalemia, and hypercalcemia may predispose patient to digoxin toxicity. Monitor electrolytes frequently during therapy. Avoid rapid changes. Supplements indicated to maintain normal serum electrolyte levels. ■ Monitor renal function. ■ Monitor digoxin levels. Draw at least 6 hours after the last dose, preferably just before the next dose. Serum digoxin levels less than 0.5 ng/mL have been associated with diminished efficacy, whereas levels above 2 ng/mL have been associated with increased toxicity without increased benefit. ■ Monitor HR and BP. ■ Baseline and periodic ECG monitoring suggested. May prolong the PR interval and cause depression of the ST segment on ECG. ■ The earliest and most frequent manifestation of digoxin toxicity in infants and children is the appearance of cardiac arrhythmias, including sinus bradycardia. ECG monitoring recommended in pediatric patients to avoid intoxication. ■ See Dose Adjustments, Precautions, and Drug/Lab Interactions.
Patient education:
Review all medications with pharmacist or physician. Capable of numerous drug interactions. ■ Laboratory monitoring of digoxin levels and renal function required. ■ Report any nausea, vomiting, persistent diarrhea, confusion, weakness, or vision disturbances.
Maternal/child:
Category C: use during pregnancy, labor, and delivery only if clearly needed. ■ Use caution during breast-feeding. Digoxin does distribute into breast milk, but the estimated exposure of the nursing infant to digoxin via breast-feeding is far below the usual infant maintenance dose. ■ Safety and effectiveness of digoxin in the control of ventricular rate in pediatric patients with atrial fibrillation have not been established. ■ Safety and effectiveness in the treatment of heart failure in pediatric patients have not been established in well-controlled studies. However, in published literature of pediatric patients with heart failure of various etiologies, treatment with digoxin has been associated with improvements in hemodynamic parameters and in clinical S/S. ■ Newborn infants display considerable variability in their tolerance to digoxin. Premature and immature infants are particularly sensitive. Dose must be reduced and individualized according to degree of maturity. ■ Carefully titrate dose based on clinical response in pediatric patients with renal disease; see Dose Adjustments.
Elderly:
Monitor carefully. Reduced dose may be indicated. Consider reduced body mass and reduced kidney function.
Drug/lab interactions
Interactions are numerous. Careful monitoring required when initiating, adjusting, or discontinuing drugs that may interact with digoxin. Monitor serum levels carefully and adjust doses as indicated. ■ Digoxin is a substrate for P-glycoprotein at the level of intestinal absorption, renal tubular secretion, and biliary-intestinal secretion. Drugs that induce or inhibit P-glycoprotein have the potential to alter digoxin pharmacokinetics. ■ Potassium-depleting diuretics (e.g., furosemide [Lasix], chlorothiazide [Diuril]) are a major contributing factor to digitalis toxicity. ■ Calcium may produce serious arrhythmias in digitalized patients, particularly with too-rapid IV administration. ■ Amiodarone (Nexterone), propafenone (Rythmol), quinine, spironolactone (Aldactone), and verapamil may increase serum digoxin concentration. Reduce digoxin dose by 15% to 30% and monitor levels. ■ Quinidine and ritonavir (Norvir) may increase serum levels. Decrease digoxin dose by 30% to 50% and monitor levels. ■ Synergistic with beta-blockers (e.g., atenolol [Tenormin], metoprolol [Lopressor]) and calcium channel blockers (e.g., verapamil, diltiazem [Cardizem]). Additive effects on AV node conduction may result in bradycardia and/or advanced or complete heart block. ■ ACE inhibitors (e.g., lisinopril [Prinivil, Zestril], enalapril [Vasotec]), angiotensin receptor blockers (e.g., valsartan [Diovan], irbesartan [Avapro]), NSAIDs (e.g., ibuprofen [Motrin, Advil], naproxen [Naprosyn, Aleve]), and COX-2 inhibitors (e.g., celecoxib [Celebrex]) may impair excretion of digoxin. ■ Coadministration with dofetilide (Tikosyn) has been associated with a higher incidence of torsades de pointes. ■ Coadministration with sotalol (Betapace) has been associated with more proarrhythmic events than when either drug was administered alone. ■ Sudden death was more common in patients receiving digoxin with dronedarone (Multaq) than when either drug was given alone. ■ Teriparatide (Forteo) transiently increases serum calcium, which may predispose patients to digoxin toxicity. ■ Succinylcholine may cause a sudden extrusion of potassium from muscle cells; may cause arrhythmias in digitalized patients. ■ Increased risk of arrhythmias with sympathomimetic amines (e.g., dopamine, epinephrine, norepinephrine). ■ Initiation of thyroid treatment may require an increase in digoxin dose. ■ No significant changes in digoxin exposure have been reported when IV digoxin is coadministered with carvedilol (Coreg), clarithromycin (Biaxin), isradipine (DynaCirc CR), losartan (Cozaar), and rifampin (Rifadin). ■ Endogenous substances of unknown composition (digoxin-like immunoreactive substances [DLIS]) can interfere with standard radioimmunoassays for digoxin. This interference usually results in a falsely elevated level but sometimes causes results to be falsely reduced. DLIS are present in up to half of all neonates and in varying percentages of pregnant women, patients with hypertrophic cardiomyopathy, patients with renal or hepatic dysfunction, and other patients who are volume expanded for any reason. Spironolactone and some traditional Chinese and Ayurvedic medicines may also interfere with different assays; see manufacturer’s prescribing information for further information. ■ See Precautions and Monitor.
Side effects
The overall incidence of adverse reactions with digoxin has been reported as 5% to 20%, with 15% to 20% of adverse reactions considered serious. Cardiac toxicity accounts for about one half, GI disturbances for about one fourth, and CNS and other toxicity for about one fourth of these adverse reactions. Cardiac: Arrhythmias (e.g., first-degree, second-degree, or third-degree heart block [including asystole]; atrial tachycardia with block; AV dissociation; accelerated junctional [nodal] rhythm; unifocal or multiform ventricular premature contractions [especially bigeminy or trigeminy]; ventricular tachycardia; and ventricular fibrillation). GI: Abdominal pain, hemorrhagic necrosis of the intestines, intestinal ischemia, nausea and vomiting. CNS: Apathy, confusion, dizziness, headache, mental disturbances (e.g., anxiety, delirium, depression, hallucinations), and weakness. Other: Anorexia, gynecomastia, rash, thrombocytopenia, and visual changes.
Toxicity can cause death. The most common manifestation of excessive dosing in pediatric patients is the appearance of cardiac arrhythmias. Conduction disturbances or supraventricular tachyarrhythmias are the most common type of arrhythmia. Ventricular arrhythmias are less common.
Overdose:
Anorexia, arrhythmias, CNS disturbances, fatigue, hyperkalemia, nausea and vomiting.
Antidote
Discontinue the drug at the first sign of toxicity, notify the physician, and place the patient on a cardiac monitor. Dosage may be decreased or discontinued. For severe toxicity, digoxin immune Fab is a specific antidote. Consider causes of toxicity (electrolyte disturbances, thyroid, concurrent medications) and correct/treat as indicated. Serum potassium must be obtained before administering potassium salts. See Precautions. Bradycardia and heart block caused by digoxin toxicity may respond to atropine. Ventricular arrhythmias may respond to lidocaine or phenytoin. A temporary pacemaker may also be used. With severe digoxin toxicity, potassium may be released from skeletal muscles, resulting in hyperkalemia. Hyperkalemia, if life-threatening, may be treated with D5W and insulin. Peritoneal dialysis or hemodialysis not effective in overdose.
Digoxin immune FAB (ovine)
(dih-JOX-in im-MYOUN fab)
Digibind, DigiFab
Antidote (digoxin intoxication)
pH 6 to 8
Usual dose
Testing for sensitivity to sheep serum and/or premedication may be indicated; see Contraindications and Monitor.
Acute toxicity in adults and pediatric patients:
Determine dose by symptoms and clinical findings. Serum concentration may not reflect actual toxicity for 6 to 12 hours. Symptoms of life-threatening toxicity due to digoxin overdose include severe arrhythmias (e.g., VT, VF), progressive bradycardia, second- or third-degree heart block not responsive to atropine, and/or serum potassium levels exceeding 5 to 5.5 mEq/L in adults and 6 mEq/L in pediatric patients.
Dose in numbers of vials based on ingested dose is calculated by dividing the body load of digoxin in milligrams by 0.5. Each vial of Digibind contains 38 mg/vial and will bind 0.5 mg digoxin. Each vial of DigiFab contains 40 mg/vial and will bind 0.5 mg of digoxin. Dose may also be based on serum digoxin levels (see package insert; has charts for adults and pediatric patients).
An initial dose of up to 20 vials has been used. 20 vials will bind approximately 50 (0.25 mg) tablets of Lanoxin and should provide adequate treatment of most life-threatening ingestions in adult and pediatric patients. If ingested substance is unknown, if serum digoxin level is not available, or if there is concern about sensitivity to the serum, consider giving 10 vials. Observe clinical response and repeat if indicated. In clinical trials of Digibind the average dose was 10 vials. A single dose may be repeated in several hours if toxicity has not reversed or appears to recur. Febrile reactions are dose related.
Toxicity in chronic therapy:
Adults:
6 vials should be adequate to reverse most cases of toxicity in adults in acute distress or if a serum digoxin concentration is not available.
Pediatric patients: Less than 20 kg:
1 vial should be adequate if signs of toxicity are present.
Dilution
Each vial must be diluted with 4 mL of SWFI (results in 9.5 mg/mL for Digibind and 10 mg/mL for DigiFab). Mix gently. May be given in this initial dilution or may be further diluted with any desired amount of NS (with Digibind, 34 mL NS/vial yields 1 mg/mL; with DigiFab, 36 mL NS/vial yields 1 mg/mL). Consider volume overload in pediatric patients when further diluting in NS. Administer to infants after initial dilution using a tuberculin syringe to deliver an accurate dose with less volume; for extremely small doses, dilute to 1 mg/mL before administration.
Filters:
Digibind:
Must be given through a 0.22-micron membrane filter.
Storage:
Refrigerate unreconstituted vials. Use reconstituted solution promptly or store in refrigerator for up to 4 hours.
Compatibility
Specific information not available. Consider specific use; consult pharmacist.
Rate of administration
Decrease the rate of infusion or discontinue temporarily if an infusion reaction occurs. Do not give as an IV bolus injection unless cardiac arrest is imminent. Be prepared to treat anaphylaxis.
Digibind:
Must be given through a 0.22-micron membrane filter. A single dose as an IV infusion equally distributed over 15 to 30 minutes.
Digifab:
A single dose as an infusion over 30 minutes.
Actions
Antigen-binding fragments (Fab) prepared from specific antidigoxin antibodies produced in sheep are isolated and purified. Fab fragments bind molecules of digoxin and make them unavailable for binding at their site of action. Freely distributed in extracellular space. Reduces the level of free digoxin in the serum. Onset of action is prompt, with improvement in symptoms of toxicity within 30 minutes. Fab-digoxin complexes are cleared by the kidney. DigiFab is also cleared in the reticuloendothelial system.
Indications and uses
Digibind:
Treatment of patients with life-threatening digoxin intoxication or overdose (digoxin).
Digifab:
Treatment of patients with life-threatening or potentially life-threatening digoxin toxicity or overdose. Not indicated for milder cases of digoxin toxicity.
All formulations:
Indicated for known suicidal or accidental consumption of fatal doses of digoxin, including ingestion of 10 mg or more of digoxin in previously healthy adults, 4 mg (or more than 0.1 mg/kg) in previously healthy pediatric patients, or ingestion causing steady-state serum concentrations greater than 10 ng/mL. ■ Indicated for chronic ingestions causing steady-state serum digoxin concentrations exceeding 6 ng/mL in adults or 4 ng/mL in pediatric patients. ■ Indicated for manifestations of life-threatening toxicity due to digoxin overdose, including severe ventricular arrhythmias (such as VT or VF), progressive bradycardia, or third-degree heart block not responsive to atropine. ■ Also indicated when potassium concentrations are above 5 to 5.5 mEq/L in adults or 6 mEq/L in pediatric patients with rapidly progressive S/S of digoxin toxicity. ■ See Precautions and Maternal/Child.
Contraindications
None known when used for specific indications. If hypersensitivity exists and treatment is necessary, premedicate with corticosteroids and diphenhydramine and prepare to treat anaphylaxis.
Precautions
Administered under the direction of the physician specialist with facilities for monitoring the patient and responding to any medical emergency. Cardiac arrest can result from ingestion of more than 10 mg digoxin by healthy adults, 4 mg digoxin by healthy pediatric patients, or serum digoxin levels above 10 ng/mL. ■ Larger doses of digoxin immune Fab act more quickly but increase the possibility of febrile or hypersensitivity reactions. ■ Use caution in impaired cardiac function. Inability to use cardiac glycosides may endanger patient. Support with dopamine or vasodilators. ■ The clinical problem may not be caused by digoxin toxicity if the patient fails to respond to digoxin immune Fab. ■ Consider that multiple drugs may have been used and are producing toxicity in suicide attempts. ■ See Monitor and Drug/Lab Interactions.
Monitor:
Although allergy testing is not required before treating life-threatening digoxin toxicity, patients allergic to ovine proteins or those who have previously received antibodies or Fab fragments produced from sheep are at risk. Determine patient response to any previous injections of serum of any type and history of any allergic-type reactions. In addition, DigiFab considers that patients with allergies to papain, chymopapain, other papaya extracts, or the pineapple enzyme bromelain may be at risk. Digibind provides the information below on sensitivity testing; that information is not included in the package insert for DigiFab. ■ Test for sensitivity if indicated. Make a 1∶100 solution by diluting 0.1 mL of reconstituted solution (10 mg/mL) with 9.9 mL sterile NS (100 mcg/mL).
Scratch test: Make a 1/4-inch skin scratch through a drop of 1∶100 dilution in NS. Inspect the site in 20 minutes. An urticarial wheal surrounded by a zone of erythema is a positive reaction.
Skin test: Inject 0.1 mL (10 mcg) of 1∶100 dilution intradermally. Inspect the site in 20 minutes. A urticarial wheal surrounded by a zone of erythema is a positive reaction. Concomitant use of antihistamines may interfere with sensitivity tests. If skin testing causes a systemic reaction, place a tourniquet above the testing site and treat anaphylaxis.
■ Serum digoxin or digitoxin concentration should be obtained before administration if at all possible. These measurements may be difficult to interpret if drawn soon after the last digitalis dose because at least 6 to 8 hours are required for equilibration of digoxin between serum and tissue. ■ Standard treatment of digoxin intoxication includes withdrawal of the intoxicating agent; correction of electrolyte disturbances (especially hyperkalemia), acid-base imbalances, and hypoxia; and treatment of cardiac arrhythmias. ■ Monitor VS, ECG, and potassium concentration frequently during and after drug administration. ■ Monitor for S/S of an acute hypersensitivity reaction (e.g., angioedema, bronchospasm with wheezing or cough, erythema, hypotension, laryngeal edema, pruritus, stridor, tachycardia, urticaria). ■ Potassium may be shifted from inside to outside the cell, causing increased renal excretion. May appear to have hyperkalemia while there is a total body deficit of potassium. When the digoxin effect is reversed, hypokalemia may develop rapidly. ■ Do not redigitalize until all Fab fragments have been eliminated from the body. May take several days. May take longer in severe renal impairment, and reintoxication may occur by release of newly unbound digoxin into the blood. ■ See Precautions and Drug/Lab Interactions.
Maternal/child:
Category C: use only when clearly indicated and benefits outweigh risks in pregnancy, breast-feeding, and infants. ■ Digibind indicates that it should be used in infants and children if more than 0.3 mg of digoxin is ingested, if serum digoxin levels are equal to or greater than 6.4 nmol/L, or if there is underlying heart disease.
Patient education:
Contact the physician immediately if S/S of a delayed hypersensitivity reaction or serum sickness occur (e.g., rash, pruritus, urticaria).
Elderly:
Consider age-related impaired renal function; monitor closely for recurrent toxicity; see Monitor.
Drug/lab interactions
Will cause a precipitous rise in total serum digoxin, but most will be bound to the Fab fragment. Will interfere with digoxin immunoassay measurements until Fab fragment is completely eliminated. ■ Catecholamines (e.g., epinephrine) may aggravate digoxin arrhythmias. ■ See skin test in Monitor.
Side effects
Acute anaphylaxis with urticaria, respiratory distress, and vascular collapse is possible. Exacerbation of congestive heart failure and low cardiac output states and increased ventricular response in atrial fibrillation may occur due to withdrawal of digoxin effects. Hypokalemia may be life threatening.
Antidote
Notify the physician of all side effects. Discontinue the drug and treat anaphylaxis immediately. Corticosteroids, epinephrine (Adrenalin [see Drug/Lab Interactions]), diphenhydramine (Benadryl), oxygen, IV fluids, vasopressors (dopamine), and ventilation equipment must always be available. Resuscitate as necessary. Treat hypokalemia cautiously when necessary. Support exacerbated cardiac conditions as necessary.
Dihydroergotamine mesylate 
(dye-hy-droh-er-GOT-ah-meen MES-ih-layt)
D.H.E. 45
Ergot alkaloid
Migraine agent
pH 3.2 to 4
Usual dose
Abort or prevent headaches:
1 mg (1 mL). May be repeated in 1 hour. No more than 2 doses (2 mg total) may be given IV in 24 hours. Do not exceed 6 mg in 1 week; see Precautions. Administration of an antiemetic (e.g., metoclopramide [Reglan] 10 mg) PO 1 hour before dihydroergotamine is recommended.
Chronic intractable headache:
0.5 mg (0.5 mL). Administer an antiemetic IV (e.g., metoclopramide) about 10 minutes before injection.
Prevention of orthostatic hypotension associated with spinal or epidural anesthesia (unlabeled):
0.5 mg (0.5 mL). Give a few minutes before anesthetic.
Pediatric dose
See Maternal/Child. Administration of an antiemetic (e.g., metoclopramide, prochlorperazine), usually PO, 1 hour before dihydroergotamine is recommended.
Pediatric patients 6 to 9 years of age:
100 to 150 mcg (0.1 to 0.15 mg).
Pediatric patients 9 to 12 years of age:
200 mcg (0.2 mg).
Pediatric patients 12 to 16 years of age:
250 to 500 mcg (0.25 to 0.5 mg).
For all age ranges, repeat up to 2 doses at 20-minute intervals if necessary. Another source suggests 250 mcg (0.25 mg) at the start of the attack. Repeat in 1 hour if necessary.
Dilution
May be given undiluted.
Storage:
Protect ampules from light and heat.
Compatibility
Specific information not available. Consider specific use; consult pharmacist.
Rate of administration
1 mg or fraction thereof over 1 minute.
Actions
An alpha-adrenergic blocking agent that causes constriction of both peripheral and cerebral blood vessels and produces depression of central vasomotor centers. Metabolized by the liver. Metabolites eliminated primarily in feces. Secreted in breast milk.
Indications and uses
To abort or prevent vascular headaches (migraine, histamine cephalalgia). Used when rapid control is desired or other routes not feasible. ■ Treatment of chronic intractable headache.
Unlabeled uses:
To prevent orthostatic hypotension associated with spinal or epidural anesthesia. Use SC to enhance heparin effects in preventing postoperative deep vein thrombosis after abdominal, thoracic, or pelvic surgeries or total hip replacement and IM or SC to treat orthostatic hypotension.
Contraindications
Breast-feeding, coronary artery disease, hepatic or renal disease, hypersensitivity, uncontrolled hypertension, peripheral vascular disease, pregnancy or women who may become pregnant, sepsis. ■ Coadministration with potent CYP3A4 inhibitors, including protease inhibitors and macrolide antibiotics, is contraindicated; see Drug/Lab Interactions.
Precautions
IM or SC use is preferred but may be given IV to obtain a more rapid effect. ■ Coadministration with potent CYP3A4 inhibitors, including protease inhibitors and macrolide antibiotics, increases the risk of vasospasm, leading to cerebral ischemia and/or ischemia of the extremities (peripheral). May be serious and/or life threatening; see Contraindications and Drug/Lab Interactions. ■ Use only when a clear diagnosis of migraine has been established. Do not exceed dosing guidelines or use for chronic daily administration; see Usual Dose.
Monitor:
Monitor vital signs; observe closely. ■ See Drug/Lab Interactions.
Patient education:
Consider birth control options. ■ Take only as directed. ■ Report ineffectiveness or an increase in frequency or severity of headaches. ■ Report chest pain, increased HR, itching, muscle pain or weakness of arms or legs, numbness or tingling of extremities, or swelling.
Maternal/child:
Category X: avoid pregnancy. See Contraindications. ■ Safety for use in pediatric patients not established. Severe side effects (e.g., extrapyramidal reactions may occur). Pretreatment with an antiemetic may be helpful. Limit pediatric use to patients who have not responded to less toxic treatment.
Elderly:
Increased risk of hypothermia and ischemic complications (e.g., cardiac, peripheral). ■ Consider age-related renal impairment.
Drug/lab interactions
Contraindicated with potent CYP3A4 inhibitors, including antifungals (e.g., itraconazole [Sporanox], ketoconazole [Nizoral]), protease inhibitors (e.g., ritonavir [Norvir], nelfinavir [Viracept], and indinavir [Crixivan]), and macrolide antibiotics (e.g., clarithromycin [Biaxin], erythromycin, and troleandomycin [TAO]); see Contraindications and Precautions. ■ Administer less potent CYP3A4 inhibitors with caution as vasospasm may occur. Less potent inhibitors include, but are not limited to, saquinavir (Invirase), nefazodone, fluconazole (Diflucan), grapefruit juice, fluoxetine (Prozac), fluvoxamine (Luvox), zileuton (Zyflo), and clotrimazole (Gyne-Lotrimin, Mycelex). ■ Opposes vasodilating effects of nitrates (e.g., nitroglycerin), decreasing their effectiveness. ■ May cause hypertensive crisis in combination with other vasopressors (e.g., epinephrine). ■ May cause peripheral vasoconstriction with ischemia and/or cyanosis with beta-adrenergic blockers (e.g., propranolol [Inderal]) and nicotine.
Side effects
Rare in therapeutic doses, but may include angina pectoris, blindness, gangrene, muscle pains, muscle weakness, nausea, numbness and tingling of the fingers and toes, pleural and retroperitoneal fibrosis, thirst, uterine bleeding, and vomiting.
Antidote
Discontinue the drug and notify the physician of any side effects. Another drug will probably be chosen if further treatment is indicated. Vasodilators (nitroprusside sodium) and CNS stimulants (e.g., caffeine and sodium benzoate) are indicated as an antidote. Heparin and low-molecular-weight dextran may be used to reduce thrombosis due to excessive vasoconstriction. Hemodialysis may be indicated. Resuscitate as necessary.
Diltiazem hydrochloride
(dill-TYE-a-zem hy-droh-KLOR-eyed)
Cardizem
Calcium channel blocker
Antiarrhythmic
pH 3.7 to 4.1
Usual dose
0.25 mg/kg of body weight initially (20 mg for the average patient). Some patients may respond to an initial dose of 0.15 mg/kg. A second dose of 0.35 mg/kg may be given in 15 minutes if needed to achieve HR reduction (25 mg for the average patient). Any additional bolus doses used to achieve an appropriate response must be individualized to each patient. Patients with PSVT will probably respond to bolus doses and may not require an infusion, but to maintain reduction in HR in patients with atrial fibrillation or atrial flutter, immediately follow with an intravenous infusion at an initial rate of 10 mg/hr. May only be used for up to 24 hours. Some patients may maintain response with an initial rate of 5 mg/hr. Infusion may be increased by 5 mg/hr increments to a maximum dose of 15 mg/hr. Discontinue infusion within 24 hours. Oral antiarrhythmic agents (e.g., digoxin, quinidine, procainamide, calcium channel blockers [e.g., diltiazem, verapamil], beta blockers [e.g., atenolol, metoprolol, propranolol]) to maintain reduced HR are usually started within 3 hours of initial bolus of diltiazem.
Dose adjustments
Specific mg/kg dose must be used for patients with low body weights. ■ Reduced dose may be indicated in impaired hepatic or renal function. ■ Dose selection should be cautious in the elderly. Reduced doses may be indicated based on potential for decreased organ function and concomitant disease or drug therapy. ■ See Drug/Lab Interactions.
Dilution
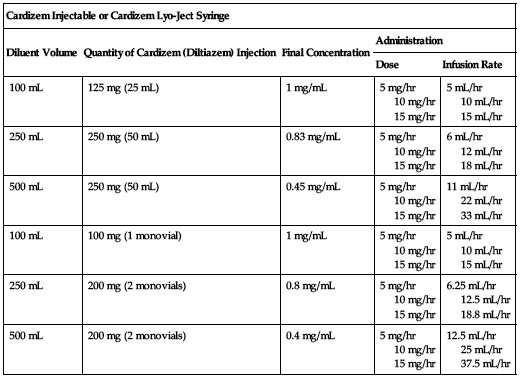
Available as a solution in 25- or 50-mg vials (5 mg/mL), as a powder with supplied diluent (Lyo-ject syringe 25 mg [5 mg/mL]), and in a piggyback monovial containing 100 mg (with transfer needle set to facilitate preparation of an infusion). Dilute according to the following charts.
| Cardizem Injectable or Cardizem Lyo-Ject Syringe | ||||
| Diluent Volume | Quantity of Cardizem (Diltiazem) Injection | Final Concentration | Administration | |
| Dose | Infusion Rate | |||
| 100 mL | 125 mg (25 mL) | 1 mg/mL | 5 mg/hr 10 mg/hr 15 mg/hr | 5 mL/hr 10 mL/hr 15 mL/hr |
| 250 mL | 250 mg (50 mL) | 0.83 mg/mL | 5 mg/hr 10 mg/hr 15 mg/hr | 6 mL/hr 12 mL/hr 18 mL/hr |
| 500 mL | 250 mg (50 mL) | 0.45 mg/mL | 5 mg/hr 10 mg/hr 15 mg/hr | 11 mL/hr 22 mL/hr 33 mL/hr |
| 100 mL | 100 mg (1 monovial) | 1 mg/mL | 5 mg/hr 10 mg/hr 15 mg/hr | 5 mL/hr 10 mL/hr 15 mL/hr |
| 250 mL | 200 mg (2 monovials) | 0.8 mg/mL | 5 mg/hr 10 mg/hr 15 mg/hr | 6.25 mL/hr 12.5 mL/hr 18.8 mL/hr |
| 500 mL | 200 mg (2 monovials) | 0.4 mg/mL | 5 mg/hr 10 mg/hr 15 mg/hr | 12.5 mL/hr 25 mL/hr 37.5 mL/hr |
IV injection:
May be given undiluted through Y-tube or three-way stopcock of tubing containing NS, D5W, or D5/1/2NS.
Infusion:
May be further diluted for infusion in any of the above solutions.
Filters:
No data available from manufacturer.
Storage:
Vials may be stored at room temperature for up to 1 month, then discarded. Refrigeration before and after dilution preferred. Use within 24 hours of dilution. Discard unused medication and/or solution. Do not freeze.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer recommends that all formulations not be mixed with any other drugs in the same container and, if possible, that they not be co-infused in the same IV line.
Cardizem Lyo-Ject Syringe is listed by the manufacturer as incompatible at the Y-site with acetazolamide (Diamox), acyclovir (Zovirax), aminophylline, ampicillin, ampicillin/sulbactam (Unasyn), diazepam (Valium), furosemide (Lasix), hydrocortisone sodium succinate (Solu-Cortef), methylprednisolone (Solu-Medrol), mezlocillin (Mezlin), nafcillin (Nallpen), phenytoin (Dilantin), rifampin (Rifadin), sodium bicarbonate. Cardizem is listed as incompatible with all of the above and insulin (regular).
Cardizem Monovial is listed by the manufacturer as incompatible at the Y-site with acetazolamide (Diamox), acyclovir (Zovirax), diazepam (Valium), furosemide (Lasix), phenytoin (Dilantin), rifampin (Rifadin).
Manufacturer lists Cardizem Lyo-Ject Syringe as compatible at the Y-site with insulin (regular) and lists the Cardizem Monovial (1 mg/mL) in NS as compatible at the Y-site with aminophylline, ampicillin, ampicillin/sulbactam (Unasyn), hydrocortisone sodium succinate (Solu-Cortef), insulin (regular), methylprednisolone (Solu-Medrol), mezlocillin (Mezlin), nafcillin (Nallpen), sodium bicarbonate.
One source lists the following compatibilities but does not differentiate formulations:
Y-site:
Acetazolamide (Diamox), acyclovir (Zovirax), albumin, amikacin, aminophylline, amphotericin B (conventional), ampicillin, ampicillin/sulbactam (Unasyn), argatroban, aztreonam (Azactam), bivalirudin (Angiomax), bumetanide, caspofungin (Cancidas), cefazolin (Ancef), cefotaxime (Claforan), cefotetan, cefoxitin (Mefoxin), ceftaroline (Teflaro), ceftazidime (Fortaz), ceftriaxone (Rocephin), cefuroxime (Zinacef), ciprofloxacin (Cipro IV), clindamycin (Cleocin), dexmedetomidine (Precedex), digoxin (Lanoxin), dobutamine, dopamine, doripenem (Doribax), doxycycline, epinephrine (Adrenalin), erythromycin (Erythrocin), esmolol (Brevibloc), fenoldopam (Corlopam), fentanyl, fluconazole (Diflucan), gentamicin, heparin, hetastarch in electrolytes (Hextend), hetastarch in NS (Hespan), hydrocortisone sodium succinate (Solu-Cortef), hydromorphone (Dilaudid), imipenem-cilastatin (Primaxin), labetalol, lidocaine, lorazepam (Ativan), meperidine (Demerol), methylprednisolone (Solu-Medrol), metoclopramide (Reglan), metoprolol (Lopressor), metronidazole (Flagyl IV), midazolam (Versed), milrinone (Primacor), morphine, multivitamins (M.V.I.), nafcillin (Nallpen), nesiritide (Natrecor), nicardipine (Cardene IV), nitroglycerin IV, nitroprusside sodium, norepinephrine (Levophed), oxacillin (Bactocill), penicillin G potassium, pentamidine, potassium chloride (KCl), potassium phosphates, procainamide (Pronestyl), ranitidine (Zantac), sodium bicarbonate, sulfamethoxazole/trimethoprim, telavancin (Vibativ), theophylline, ticarcillin/clavulanate (Timentin), tobramycin, vancomycin, vasopressin, vecuronium.
Rate of administration
IV injection:
Each single dose equally distributed over 2 minutes.
Infusion:
5 mg to 15 mg/hr based on patient response. See charts under Dilution for diluent, dose, and infusion rate information. Use of a metriset (60 gtt/min) required; volumetric infusion pump preferred.
Actions
Directly inhibits the influx of calcium ions through slow channels during membrane depolarization of cardiac and vascular smooth muscle. Effective in supraventricular tachycardias because it slows conduction through the AV node, prolongs the effective refractory period, reduces ventricular rates, and helps to prevent embolic complications. Also slows conduction through the SA node. Prevents re-entry phenomena through the AV node. Reduces HR (10% with a single dose, 20% at peak effectiveness), systolic and diastolic BP, systemic vascular resistance, pulmonary artery systolic and diastolic BPs, and coronary vascular resistance with no significant effect on contractility, left ventricular end diastolic pressure, right atrial pressure, or pulmonary capillary wedge pressure. Increases cardiac output and stroke volume. Has little or no effect on normal AV nodal conduction at normal HRs. Produces less myocardial depression than verapamil. Effective within 3 minutes; maximum effect should occur within 2 to 7 minutes and last for 1 to 3 hours. 70% to 80% bound to plasma proteins. Metabolized in the liver. Half-life is approximately 3.4 hours following a bolus injection and increases to 4.1 to 4.9 hours with continuous infusion. Excreted in urine and bile. Secreted in breast milk.
Indications and uses
Temporary control of rapid ventricular rate in atrial fibrillation or atrial flutter unless associated with an accessory bypass tract (e.g., Wolff-Parkinson-White syndrome or short PR syndrome). ■ Rapid conversion of paroxysmal supraventricular tachycardia (PSVT) to normal sinus rhythm including AV nodal re-entrant tachycardias and reciprocating tachycardias associated with an extranodal accessory pathway (e.g., Wolff-Parkinson-White syndrome or short PR syndrome).
Contraindications
Atrial fibrillation or flutter when associated with an accessory bypass tract (e.g., Wolff-Parkinson-White or short PR syndrome), cardiogenic shock, congestive heart failure (severe) unless secondary to supraventricular tachyarrhythmia treatable with diltiazem, known sensitivity to diltiazem, second- or third-degree AV block or sick sinus syndrome (unless functioning ventricular pacemaker in place), severe hypotension, patients receiving IV beta-adrenergic blocking agents (e.g., atenolol [Tenormin], propranolol [Inderal]) within 2 to 4 hours, ventricular tachycardia. Not recommended for wide QRS tachycardias of uncertain origin or for tachycardias induced by drugs or poisons.
Precautions
For short-term use only. ■ Initial administration of IV diltiazem should take place in a facility with adequate personnel, equipment, and supplies to monitor the patient and respond to any medical emergency. ■ While diltiazem will effectively decrease HR, cardioversion will probably be required to convert atrial fibrillation or atrial flutter to a normal sinus rhythm. ■ Valsalva maneuver recommended before use of diltiazem in all paroxysmal supraventricular tachycardias if clinically appropriate. ■ Use IV diltiazem with caution in patients with pre-existing impaired ventricular function (e.g., congestive heart failure, acute myocardial infarction, or pulmonary congestion documented by x-ray); may exacerbate disease. Use of oral diltiazem in these patients is contraindicated. ■ May cause second- or third-degree AV block in sinus rhythm; discontinue diltiazem if AV block occurs. ■ Can cause life-threatening tachycardia with severe hypotension in atrial fibrillation or flutter in patients with an accessory bypass tract and periods of asystole in patients with sick sinus syndrome. ■ Use with caution in impaired renal or hepatic function. ■ Ventricular premature beats (VPBs) may occur on conversion of PSVT to sinus rhythm; considered to have no clinical significance. ■ Continue regular dosing on day of OR and thereafter unless otherwise specified by physician. If discontinued, may cause severe angina or MI.
Monitor:
Accurate pretreatment diagnosis differentiating wide-complex QRS tachycardia of supraventricular origin from ventricular origin is imperative. ■ ECG monitoring during administration preferred; must be available. ■ Monitor BP and HR closely. ■ Emergency resuscitation drugs and equipment must always be available. ■ See Drug/Lab Interactions.
Maternal/child:
Category C: large doses (5 to 10 times mg/kg dose) have resulted in embryo and fetal death and skeletal abnormalities in animals. ■ Discontinue breast-feeding. ■ Safety and effectiveness for use in pediatric patients not established.
Elderly:
See Dose Adjustments. ■ Half-life may be prolonged. ■ May cause tinnitus.
Drug/lab interactions
Do not give concomitantly (within a few hours) with IV beta-adrenergic blocking agents (e.g., atenolol [Tenormin], propranolol [Inderal]); see Contraindications. May result in bradycardia, AV block, and/or depression of contractility. Use extreme caution if these drugs are administered orally or if patient has received before admission; usually tolerated. ■ May result in additive effects with any agent known to affect cardiac contractility and/or SA or AV node conduction (e.g., digoxin [Lanoxin], disopyramide [Norpace], procainamide [Pronestyl], beta-blockers [e.g., propranolol], quinidine). ■ Is used with digoxin, but monitor for excessive slowing of HR and/or AV block. ■ Coadministration with amiodarone (Nexterone) may result in bradycardia and decreased cardiac output. Monitor closely. ■ May increase effects of certain benzodiazepines (e.g., midazolam [Versed], triazolam [Halcion]), buspirone (BuSpar), methylprednisolone (Solu-Medrol). ■ May increase serum concentrations of digoxin, HMG-CoA reductase inhibitors (e.g., atorvastatin [Lipitor], simvastatin [Zocor]), imipramine (Tofranil), sirolimus (Rapamune), and tacrolimus (Prograf). Monitor serum levels and/or monitor for S/S of toxicity. ■ May potentiate anesthetics; titrate both drugs carefully. ■ May decrease metabolism and increase serum concentrations and toxicity of drugs metabolized by the cytochrome P450 enzyme system (e.g., amlodipine [Norvasc], carbamazepine [Tegretol], cyclosporine [Sandimmune], quinidine, theophylline, valproate [Depacon]). ■ Metabolism may be decreased and serum concentrations increased by cimetidine (Tagamet) and ranitidine (Zantac). ■ Metabolism may be increased and serum concentrations decreased by rifampin (Rifadin). Adjust dose as needed. ■ May increase moricizine (Ethmozine) serum concentrations. Moricizine may decrease diltiazem concentrations. ■ May increase nifedipine (Procardia) serum concentrations; nifedipine may increase diltiazem serum concentrations. ■ Variable effects when administered with lithium. Has caused decreased effectiveness of lithium and may cause neurotoxicity. ■ Any drug metabolized in the liver may cause competitive inhibition of metabolism (e.g., insulin).
Side effects
Arrhythmia (junctional rhythm or isorhythmic dissociation), flushing, hypotension (asymptomatic and symptomatic), and injection site reactions (burning, itching) occurred most frequently and were most often mild and transient but could have serious potential. Amblyopia, asthenia, atrial flutter, AV block (first- or second-degree), bradycardia, chest pain, congestive heart failure, constipation, dizziness, dry mouth, dyspnea, edema, elevated alkaline phosphatase and AST, headache, hyperuricemia, nausea, paresthesia, pruritus, sinus node dysfunction, sinus pause, skin eruptions (including rare reports of exfoliative dermatitis or Stevens-Johnson syndrome), sweating, syncope, ventricular arrhythmias, ventricular fibrillation, ventricular tachycardia, and vomiting have occurred.
Antidote
Discontinue diltiazem if a high-degree AV block occurs in sinus rhythm. Notify physician promptly of all side effects. Treatment will depend on clinical situation; maintain IV fluids as indicated. Rapid ventricular response in atrial flutter/fibrillation should respond to cardioversion, procainamide, and/or lidocaine. Treat bradycardia, AV block, and asystole with standard AHA protocol (atropine, isoproterenol, pacing). Treat cardiac failure with inotropic agents (isoproterenol, dopamine, or dobutamine) and diuretics. Calcium chloride will reverse effects of verapamil; may be useful with diltiazem. Dopamine or norepinephrine (levarterenol) and Trendelenburg position should reverse hypotension. Treat hypersensitivity reactions or resuscitate as necessary. Not removed by hemodialysis.
Dinutuximab 
(dye-new-TUX-ih-mab)
Unituxin
Antineoplastic
(monoclonal antibody)
pH 6.8
Usual dose
Verify adequate hematologic, respiratory, hepatic, and renal function before initiating each course of dinutuximab; see Monitor.
Administer required premedication and hydration before initiation of each dinutuximab infusion.
Hydration:
Administer NS 10 mL/kg as an IV infusion over 1 hour just before initiating each dinutuximab infusion.
Premedication:
Analgesics:
Immediately before initiating dinutuximab infusion, administer morphine sulfate 50 mcg/kg IV. Follow with a morphine sulfate drip at an infusion rate of 20 to 50 mcg/kg/hr during and for 2 hours after completion of dinutuximab. Additional 25 to 50 mcg/kg IV doses of morphine may be administered as needed for pain up to once every 2 hours followed by an increase in the morphine infusion rate in clinically stable patients. Consider using fentanyl or hydromorphone (Dilaudid) if morphine is not tolerated. If pain is not managed adequately with opioids, consider use of gabapentin (Neurotin) or lidocaine in conjunction with IV morphine.
Antihistamines:
20 minutes before initiating dinutuximab infusion, administer an antihistamine such as diphenhydramine (Benadryl) 0.5 to 1 mg/kg (maximum dose of 50 mg) IV over 10 to 15 minutes. Repeat every 4 to 6 hours as tolerated during the dinutuximab infusion.
Antipyretics:
20 minutes before initiating dinutuximab infusion, administer acetaminophen 10 to 15 mg/kg (maximum dose 650 mg). Repeat every 4 to 6 hours as needed for fever or pain. Administer ibuprofen 5 to 10 mg/kg every 6 hours as needed for control of persistent fever or pain.
Dinutuximab:
17.5 mg/M2/day as an IV infusion over 10 to 20 hours for 4 consecutive days for a maximum of 5 cycles. See Rate of Administration.
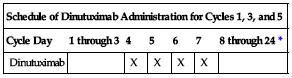
| Schedule of Dinutuximab Administration for Cycles 1, 3, and 5 | ||||||
| Cycle Day | 1 through 3 | 4 | 5 | 6 | 7 | 8 through 24* |
| Dinutuximab | X | X | X | X | ||
| Schedule of Dinutuximab Administration for Cycles 2 and 4 | ||||||
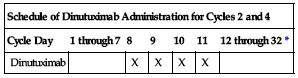
| Cycle Day | 1 through 7 | 8 | 9 | 10 | 11 | 12 through 32* |
| Dinutuximab | X | X | X | X | ||
Dose adjustments
Manage adverse reactions by infusion interruption, infusion rate reduction, dose reduction, or permanent discontinuation of dinutuximab as outlined in the following charts; see Rate of Administration, Monitor, and Antidote.
| Dinutuximab Dose Modification for Infusion-Related Reactions | ||
| Mild to moderate adverse reactions (e.g., transient rash, fever, chills, localized urticaria) that respond promptly to symptomatic treatment | Onset of reaction | Reduce dinutuximab infusion rate to 50% of the previous rate and monitor closely. |
| After resolution | Gradually increase infusion rate up to a maximum rate of 1.75 mg/M2/hr. | |
| Prolonged or severe adverse reactions (e.g., mild bronchospasm without other symptoms, angioedema that does not affect the airway) | Onset of reaction | Immediately interrupt dinutuximab. |
| After resolution | If S/S resolve rapidly, resume dinutuximab infusion at 50% of the previous rate and monitor closely. | |
| First recurrence | Discontinue dinutuximab until the following day. If symptoms resolve and continued treatment is warranted, premedicate with hydrocortisone 1 mg/kg IV (maximum dose 50 mg) and administer dinutuximab at a rate of 0.875 mg/M2/hr in an intensive care unit. | |
| Second recurrence | Permanently discontinue dinutuximab. | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


