. DIAGNOSTIC ORDERS
Venipuncture42
Capillary Puncture50
Throat Specimens52
Wound Specimens53
Urine Specimens53
Fecal Specimens57
Sputum Specimens57
Diagnostic Testing58
Other Diagnostic Tests75
SPECIMEN COLLECTION
As a medical assistant (MA), you will either collect specimens (e.g., blood, throat, wound specimens for culture) or instruct patients in how to collect the specimens themselves (e.g., urine, feces).
You may be responsible for testing these specimens in the office laboratory, or you may be responsible for properly labeling, storing, and/or transporting these specimens to an outside laboratory for testing.
Proper collection of specimens is imperative. Improperly collected specimens can yield poor or inconclusive results. When collecting and processing specimens, perform the following:
• Always use standard precautions.
• Be familiar with specimen storage (e.g., refrigeration, chemical additives) and transportation procedures.
• Learn how to fill out a laboratory requisition form for the particular laboratory your office uses to perform outside testing.
Venipuncture for collecting venous blood for testing is usually carried out using a vein on the inside of the elbow. The blood may be collected in evacuated tubes or in a syringe. If a syringe is used, the blood is transferred to evacuated tubes that often contain additives.
Modified from Hunt SA: Saunders fundamentals of medical assisting, St Louis, 2007, Saunders. | ||||
| Vacutainer Colors* | Additives | Laboratory Use | Handling Instructions | |
|---|---|---|---|---|
| Yellow | SPS (anticoagulant) | Blood or body fluid cultures | Ensure sterility on collection. | |
| Plain red | None | Serum testing, immunologie findings, serologie findings, blood bank, chemistry | Allow to clot at least 40 min; centrifuge; access to clot for blood bank testing. | |
| Red and | Gray (marbled) † | None but contains silica particles to enhance clot formation | Serum testing | Allow to clot at least 40 min; centrifuge and analyze; no access to clot. |
| Light blue | Sodium citrate (anticoagulant) | Coagulation testing | Centrifuge immediately on collection; or separate plasma and refrigerate; test within 4 hr or freeze. | |
| Green | Sodium/lithium heparin (anticoagulant) | Chemistry testing | Centrifuge immediately; separate to analyze. | |
| Lavender | EDTA (anticoagulant) | Hematologic testing | Mix well; analyze within 4-8 hr of collection. | |
| Gray | Potassium oxalate/sodium fluoride (anticoagulant) | Chemistry testing; glucose determinations (especially when delay in testing is anticipated); alcohol levels | Centrifuge and separate to analyze. | |
| EDTA, Ethylenediaminetetraacetic acid; SPS, sodium polyanethole suffonate. | ||||
| *Stopper colors are based on Becton-Dickinson tubes. | ||||
| †The manufacturer recommends treating this tube as one containing additives and using it in the order listed above, but some laboratories consider it a tube without additives and use it immediately after a plain red-capped tube. | ||||
1. Have appropriate types and sizes of tubes (for every 1 ml of serum needed, draw 2.5 ml of whole blood).
2. Wash hands and change gloves between each procedure.
3. Properly identify the patient.
4. Review the test requisition.
5. Assemble all the necessary equipment before beginning the draw. Be sure to have extra tubes available in case a tube has no vacuum.
6. Use an alternative cleaning method for the draw site, rather than an alcohol pad, if a blood alcohol level is to be drawn. Hydrogen peroxide or soap and water may be used.
7. To avoid damaging blood cells, do not use a needle with a gauge smaller than 23.
1. Wash your hands.
2. Prepare supplies and equipment per the laboratory slip or encounter form, including the gathering all of the appropriate tubes for the tests ordered (i.e., color coded in the correct order of draw); securely screw the posterior needle into the holder, and open an alcohol pad.
3. Identify the patient, explain the procedure, and confirm the requisition and any special preparations (e.g., fasting). Arrange collection tubes in the proper order of draw.
5. Put on gloves, cleanse the site with alcohol, and dry with a gauze square.
6. Pick up the holder in your dominant hand, and place the first tube in the holder without pushing it onto the needle.
7. Remove the needle cover.
8. Stabilize and penetrate the vein using a 15- to 20-degree angle in one quick motion.
9. Allow the holder to rest on your fingers, push the tube onto the needle, and allow it to fill from the vacuum in the tube.
10. Withdraw the tube when full and change tubes as necessary with the holder remaining in place.
11. When the last tube begins filling, release the tourniquet.
12. Remove the last tube from the holder, remove the needle from the arm, and place a gauze square over the puncture to stop the bleeding. Tell the patient to hold the gauze firmly over the puncture site and to keep the arm straight.
13. Dispose of the needle in a rigid biohazard container, gently rotating any tubes with additives; label the tubes or place preprinted bar-coded labels on the tubes.
14. When the bleeding has stopped, put a bandage on the puncture site and discard the gauze squares and gloves in the biohazard container.
15. Wash your hands; document the results.
When collecting blood using the butterfly needle, prepare equipment, greet and explain the procedure to the patient, don gloves, and assess the patient’s veins as follows:
• Pick up the prepared holder or syringe with the butterfly needle and tubing attached, and remove needle cover from butterfly needle.
• Attach the butterfly unit to the needle holder.
• Hold the wings in your dominant hand with the needle at a 15-degree angle; stabilize the vein, and puncture the vein with one quick motion.
• Allow the needle to rest in the vein supported by the wings. Gently move up or down if necessary until the needle rests flat.
If using evacuated tubes, push the first tube onto the needle and allow it to fill by vacuum action. If using a syringe, slowly pull back the plunger until the syringe has filled with the desired amount of blood.
If you draw blood into a syringe, transfer it to evacuated tubes as previously described.
1. Prepare equipment, greet and identify the patient, explain the procedure, assess the patient’s veins, put on gloves, and prepare the site as described in the procedure for venipuncture using the evacuated-tube method.
3. Remove the needle cover from the syringe.
4. Holding the syringe in your dominant hand at a 15-degree angle, stabilize the vein, and penetrate the vein with one quick motion.
5. Slowly pull back on the plunger of the syringe until blood fills the syringe. Keep pulling slowly until you have obtained enough blood for the tests ordered.
6. Release the tourniquet, remove the needle from the patient’s arm, apply pressure to the puncture site with a gauze square, and ask the patient to hold the gauze in place firmly with the arm straight.
7. Transfer blood to the evacuated tubes by inserting the needle through the rubber stopper and allowing the vacuum to pull the blood into the tube. To avoid a needle-stick injury, do not hold tubes while inserting the needle.
8. Gently rotate any tubes with additives and label.
9. When the bleeding has stopped, apply a bandage.
10. Remove gloves and discard in biohazard waste container, wash your hands, and document.
IF YOU HAVE PROBLEMS FINDING THE VEIN
If you do not obtain blood after making the puncture, gently palpate over the needle to determine whether the tip of the needle is above the vein, is beside the vein, or has penetrated through the vein. Based on your assessment, make one or two attempts to enter the vein to avoid having to start again, but do not dig around excessively; doing so can cause a hematoma. The following figures illustrate a summary of problems that can prevent blood from entering the evacuated tube or syringe:
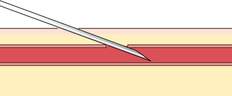 |
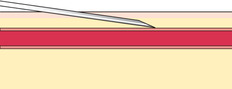 |
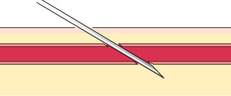 |
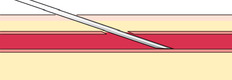 |
 |
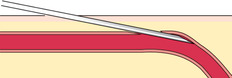 |
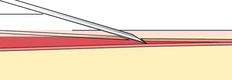 |
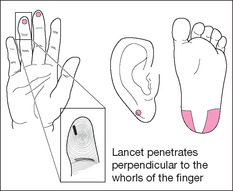
A capillary puncture is performed either on a finger (in older children and adults) or on a heel (in infants). Capillary punctures are performed to obtain a small amount of blood with which to perform blood tests including frequent blood sugar tests for patients with diabetes.
Heel sticks are performed on children who have not started to walk. Once a child begins to walk, the heel becomes callused, and any capillary blood necessary should be drawn from the finger. Use the side of the heel to avoid damage to the calcaneus bone. Blood will flow more freely if the heel is warmed using a towel moistened with warm water for 5 to 10 minutes before the procedure. If necessary, the earlobe can also be used to obtain capillary blood.
 |
1. Wash your hands; assemble supplies.
2. Greet and identify the patient; explain the procedure.
3. Cleanse the finger selected for puncture using an alcohol pad.
4. Put on gloves.
5. Twist the small knot off a manual lancet, or prepare an automatic safety lancet. Safety lancets are almost universal today.
6. Hold the finger in a downward position.
7. Without touching the area you have cleaned, make a puncture with the sterile lancet perpendicular to the fingerprint swirls on the lateral surface of the finger.
8. Drop lancet into sharps container.
9. Allow blood to flow. Wipe away the first drop of blood.
10. Collect a hanging drop of blood on pad of a test strip, or collect a specimen in a microcollection device.
11. Place a sterile gauze square over the puncture site and apply pressure. When the bleeding stops, apply a bandage.
12. Perform tests.
13. Remove your gloves and discard with soiled gauze in a biohazard waste container.
14. Wash your hands; document the results.
1. Wash your hands, assemble equipment, and open the alcohol pad and sterile gauze square.
2. Identify the infant, greet the infant’s caregiver, and explain the procedure.
4. Clean the area of the heel where the puncture will be made.
5. Put on gloves and prepare a pediatric lancet, which does not penetrate as deeply as an adult lancet.
6. Firmly grasp the infant’s heel and make the puncture on the side of the heel.
7. Dispose of the lancet in a rigid biohazard container.
8. Let blood flow and wipe away the first drop.
9. Collect the specimen in appropriate microcollection container. If you are collecting a specimen to be tested for phenylketonuria (PKU), ensure that each circle of the test card is completely saturated with blood.
10. Place the sterile gauze over the puncture and apply pressure until the bleeding stops; then cover with a bandage.
11. Perform tests.
12. Remove gloves and dispose of them along with gauze in a biohazard waste container.
13. Wash your hands; document the results.
THROAT SPECIMENS
Throat specimens are collected to test for group A beta hemolytic Streptococcus bacteria, the primary cause of bacterial pharyngitis (sore throat) in North America. Treating strep infections is important because untreated, strep infections can lead to bacterial endocarditis, rheumatic fever, or acute glomerulonephritis.
When collecting a throat culture, perform the following procedures:
• Use a tongue depressor to hold down the tongue.
• Obtain a specimen from the back of the throat without touching the tongue or teeth with the sterile swab.
• Swab the back of the throat in a figure-8 motion.
• Obtain the specimen from any visible pus on the tonsils.
One swab may be used to perform a rapid strep test in the office. If the test is negative, the other swab will be either cultured in the office or sent to an outside laboratory. To transport a specimen, place the swab into a container with transport medium (such as a Culturette), and place the cap on the container. Firmly squeeze the container to release the transport medium. Be sure to label the specimen and fill out any necessary requisitions.
WOUND SPECIMENS
A wound may contain aerobic or anaerobic bacteria. Deep wounds especially are likely to contain anaerobic bacteria, which flourish under conditions with no oxygen.
• Wound specimens should be collected from the wound with the correct type of swab for the culture needed. The physician will collect the specimen while wearing sterile gloves.
• Each swab is immediately placed in a culture tube, and the media-transport ampule should be crushed by squeezing the sides of the tube firmly.
• Discard all the waste in the biohazard waste container, remove and discard gloves, and wash your hands; document the results.
URINE SPECIMENS
Urine specimens can be collected in a number of different ways for many different purposes.
Random urine specimens are used for pregnancy-confirmation tests and general tests as part of a routine physical.
To collect a random sample, label a specimen cup, instruct the patient to void approximately 50 to 100 ml of urine into the specimen cup, and replace the cover tightly. Wear gloves to handle the specimen.
CLEAN-CATCH MIDSTREAM
A clean-catch midstream specimen is the method of choice for microscopic examination, as well as for pregnancy testing and urinalysis.
To obtain a clean-catch midstream sample, you need to instruct the patient in how to properly clean his or her genital area and collect the urine without contaminating the collection device. Instruct the patient to wash his or her hands before collecting the urine specimen and avoid touching the inside of the lid or container. Tell the patient where to take or leave the specimen.
Female patients perform the following procedure to collect a clean-catch midstream sample:




• Wash your hands and open the cup, placing the inside of the lid up.
• Spread the labia and clean the genital area from front to back using each cleansing towel only once. Clean down the left side and discard the towel. Next, clean down the right side and discard the towel. Then clean down the middle and discard the towel.
• Continue to hold the labia apart and void a small amount into the toilet. Then void into the specimen cup until it is about half full. Void any remaining urine into the toilet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
