Chapter 29 Cryptococcosis and other fungal infections (histoplasmosis and coccidioidomycosis) in HIV-infected patients
Cryptococcus
Microbiology
Cryptococcus neoformans is a round or oval yeast (4–6 μm in diameter), surrounded by a capsule that can be up to 30 μm thick. The organism grows readily on fungal or bacterial culture media and is usually detectable within 1 week after inoculation, although in some circumstances up to 4 weeks are required for growth. It grows well at 37°C, does not form pseudomycelin on cornmeal or rice-Tween agar, and hydrolyzes urea, a property that allows rapid presumptive identification [1].
On the basis of antigenic differences in the capsule, biochemical use of nutrients, and distinct DNA base sequences, four serotypes (A, B, C, and D) of Cryptococcus have been delineated. Serotypes A and D are classified as C. neoformans var. neoformans, and serotypes B and C are classified as C. neoformans var. gatii [2]. Cryptococcus neoformans var. neoformans is the major cause of cryptococcal disease worldwide. Serotype A is the most common serotype infecting AIDS patients [3, 4].
Epidemiology
Before the onset of the AIDS epidemic, cryptococcal infection occurred in a small number of immunocompetent individuals, but most often in patients with a compromised immune system, such as diabetics, transplant recipients, patients with lymphoma, or patients requiring chronic steroid therapy [5]. In the AIDS era, cryptococcal disease became a leading opportunistic infection, affecting 6–10% of HIV-infected patients [6–10]. It is the third most common central nervous system (CNS) disorder in AIDS patients after toxoplasmosis and HIV dementia [11].
In the past decade the incidence of disease caused by C. neoformans in developed countries has decreased dramatically, in part due to more widespread use of azole therapy [12], but primarily resulting from the introduction of highly active antiretroviral therapy (HAART). Surveillance and clinical studies in the USA and Europe have shown a 2- to 10-fold decrease in the incidence of cryptococcosis since the introduction of HAART [9, 11, 13, 14]. Demographic characteristics of patients with cryptococcosis show that poor access to medical care is a major risk factor for developing cryptococcal disease in the HAART era [14–17].
However, in areas of the world with limited access to ART, cryptococcosis remains a major cause of morbidity and mortality. It is estimated that there are approximately 1 million cases of HIV-associated cryptococcosis annually, the majority occurring in resource-limited settings [18]. Studies from sub-Saharan Africa and Southeast Asia show that up to one-third of patients infected with HIV develop cryptococcal disease [19–21], which is a major cause of death among HIV-infected patients [22]. Despite antifungal therapy, mortality from cryptococcal disease in these settings is exceedingly high [18, 22, 23], with an average survival of < 6 months after diagnosis [24, 25]. In sub-Saharan Africa, the estimated mortality attributed to cryptococcal meningoencephalitis (~ 500,000 deaths per year) is higher that the mortality attributed to tuberculosis (350,000 deaths/year) [18]. Poor outcomes may be related to the late presentation of disease and suboptimal management of raised intracranial pressure.
Natural history, pathogenesis, and pathology
Cryptococcus neoformans infection is acquired from the environment. Because it is ubiquitous in soil and dust, exposure to the organisms is practically unavoidable. The organism, most likely in an encapsulated form, is inhaled into the lungs and deposited in the small airways. Once there, yeast multiply and compress the surrounding tissue but cause little damage, and pulmonary infection is often asymptomatic. Indeed, in 85–95% of patients with cryptococcal meningoencephalitis, no evidence of pneumonitis is present [7, 26]. The organism has a strong propensity for dissemination to the CNS but also may infect skin, bone, and the genitourinary tract.
Clinical features
The onset of cryptococcal disease usually is insidious. The most common symptoms are headache, malaise, and a prolonged febrile prodrome that may be indistinguishable from symptoms caused by other opportunistic infections [7, 26–28]. The median time between the onset of symptoms and the diagnosis of cryptococcal disease is 30 days [7, 26, 27]. Diagnosis is often delayed by the waxing and waning course of the disease and the absence of specific symptoms.
Cryptococcal Meningoencephalitis
CNS involvement is the most serious and common manifestation of cryptococcal disease in HIV-infected patients. Over 75% of HIV-associated cryptococcal meningoencephalitis occurs in patients with CD4 counts < 50 cells/mm3. In general, CNS disease presents as a chronic meningoencephalitis, with headaches, nausea, and photophobia, but occasionally can present with focal neurologic signs associated with mass lesions (cryptococcomas). In a prospective study conducted in Uganda, 20% of HIV-infected patients presenting with headaches were diagnosed with cryptococcal meningoencephalitis [29]. Classic signs of meningeal irritation are uncommon. HIV-infected patients presenting with seizures, altered mental status, psychosis, and dementia should be evaluated for cryptococcal meningoencephalitis. Pulmonary or disseminated disease may be associated, but usually CNS disease presents in isolation.
The physical examination in patients with cryptococcal disease is non-specific, with fevers in approximately half the cases, but less than one-third with nuchal rigidity or other neurologic deficits [7]. Altered mental status, which is the most important predictor of poor outcome, is present in 20–30% of patients with cryptococcal disease. Papilledema is seen in < 10% of patients. Raised, sometimes umbilicated, typical skin lesions that resemble those caused by molluscum contagiosum or Penicillium marneffei are reported in 3–10% of patients with cryptococcal meningoencephalitis.
Immune reconstitution syndrome (IRIS) occurs in 8-49% of patients with cryptococcosis treated with ART [30]. The syndrome is characterized by a paradoxical clinical deterioration resulting from an exuberant inflammatory response that may manifest itself as worsening or new lymphadenopathy, mediastinitis, pneumonitis, new headache and stiff neck, CNS lesions, increased intracranial pressure, or subcutaneous abscesses [30–35]. The onset of IRIS usually occurs between the first and tenth month of ART initiation [30]. Several factors have been associated with the risk of IRIS, including high baseline viral load, initiation of ART during induction therapy, rapid rise in CD4 count, high cryptococcal titers, and lack of CSF inflammation at baseline [30, 36–38]. Usually C. neoformans cultures are negative.
Patient evaluation, diagnosis, and differential diagnosis
Cryptococcal meningoencephalitis is by far the most common manifestation of cryptococcosis in HIV-infected patients. The most common symptoms are fever, malaise, and worsening headaches over the course of a few weeks, but the indolent and non-specific presentation requires a high index of suspicion to diagnose the disease in a timely manner. Although the presentation is subacute, and not usually suggestive of bacterial meningoencephalitis, in a study from Malawi, 10% of suspected bacterial meningoencephalitis cases turned out to be cryptococcal meningoencephalitis [39]. The CD4 count can help guide the work-up and differential diagnosis. While cryptococcal meningoencephalitis is rare in patients with a CD4 count > 100 cells/mm3, it is the leading diagnosis for HIV-infected patients with low CD4 counts presenting with fever and headache.
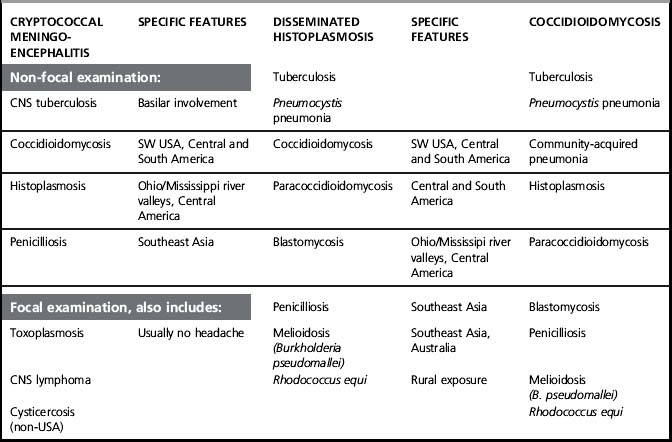
The differential diagnosis depends to some extent on local epidemiology. Central nervous system (CNS) tuberculosis (TB) is a major concern in patients from TB-endemic areas. Other considerations include chronic fungal infections such as penicillosis in patients from Southeast Asia and histoplasmosis or coccidioidomycosis in patients from Latin America. In patients who present with focal neurologic deficits or seizures, the differential diagnosis can also include toxoplasmosis, CNS lymphoma, and other non-HIV related conditions, such as cysticercosis or CNS malignancy. Despite an extensive differential diagnosis, the diagnosis of cryptococcal meningoencephalitis is generally straightforward due to the characteristic elevation in intracranial pressure and the high sensitivity and specificity of cryptococcal antigen tests (Table 29.1).
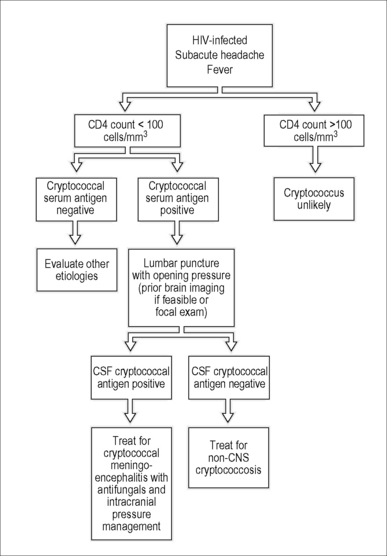
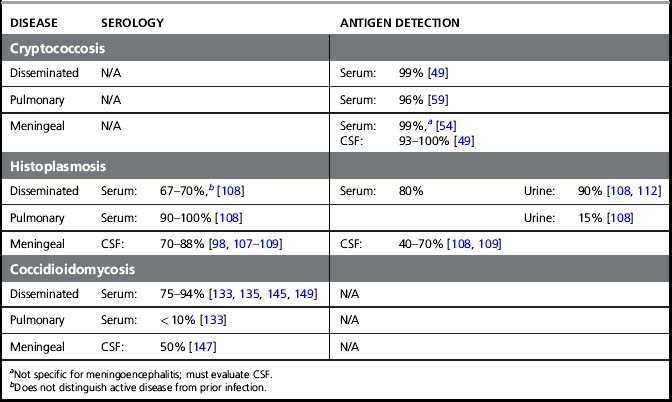
If available, the serum cryptococal antigen (CRAG) test is an excellent starting point for the evaluation of cryptococcal meningoencephalitis. Serum CRAG tests are positive in over 99% of patients with cryptococcosis and can be used to rule out cryptococcal meningoencephalitis in HIV-infected patients with a fever and a headache (Fig. 29.1) [40]. In the USA, a lumbar puncture is recommended if the serum CRAG is positive to evaluate for the presence of CNS involvement and to measure intracranial pressure [41]. However, in resource-limited settings lumbar punctures may only be practically available for patients with advanced disease. In such scenarios, it is appropriate to screen ambulatory HIV-infected patients presenting with headaches with a serum CRAG and a chest radiograph to rule out tuberculosis. Patients with a positive CRAG, negative chest radiograph, and no focal neurologic signs, headache, or altered consciousness can be successfully treated with oral fluconazole. In Uganda, screening with serum CRAG detected cryptococcal infection 20 days before the onset of frank meningoencephalitis, and early therapy with fluconazole reduced mortality rates and was cost-effective [29, 42]. However, patients with altered mental status or focal neurologic signs must have a lumbar puncture performed to confirm the diagnosis and to appropriately manage elevated intracranial pressure [41]. The CSF CRAG has high sensitivity (93–99%) and specificity (93–98%) [7, 26, 43] and can expedite or exclude the diagnosis of cryptococcal meningoencephalitis (Table 29.2).
The CSF profile of HIV-infected patients with cryptococcal meningoencephalitis is normal in 25–50% of patients [7, 26, 44–46]. Over 50% of patients have a low CSF white blood count (< 20/mm3), and the India ink preparation is positive in 74–88% of cases [7, 26, 45–47]. Interpretation of the India ink stain should be performed by an experienced technician since false-positive tests have been reported. After staining, C. neoformans is visible as round cells 4–6 μm in diameter surrounded by a characteristic thick polysaccharide capsule (Fig. 29.2). Budding forms can usually be detected with viable C. neoformans.
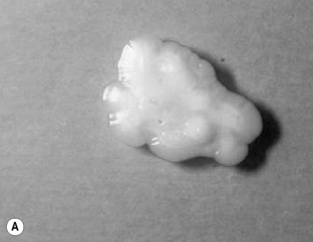
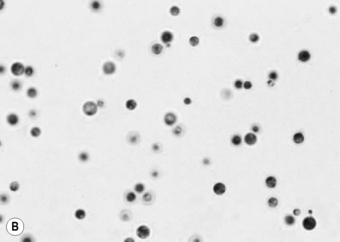
Figure 29.2 (A) Mature Cryptococcus neoformans colony. (B) Microscopic appearance of C. neoformans.
Photographs courtesy of Dr William Merz, Department of Pathology, Johns Hopkins University School of Medicine.
The gold standard diagnostic test for cryptococcal meningoencephalitis is a positive CSF culture for C. neoformans. CSF fungal cultures require a minimum of 2 mL to obtain maximum sensitivity. By definition, 100% of cases of cryptococcal meningoencephalitis have positive CSF fungal cultures. Approximately 50–80% of HIV-infected patients with cryptococcal meningoencephalitis have positive blood cultures for C. neoformans [27, 47, 48].
Prognosis
Left untreated, cryptococcal meningoencephalitis is uniformly fatal [29]. Although survival in HIV-infected patients with cryptococcal meningoencephalitis has improved with better therapeutic management and ART, 10–20% of patients die or fail therapy despite access to tertiary care [28, 47]. In areas with limited medical resources, mortality rates are even higher [22, 25, 33, 48]. In the post-HAART era in Uganda, 20% of hospitalized patients with CSF-confirmed cryptococcal meningoencephalitis died within the first two weeks of therapy and only 41% survive 6 months [25]. The most important baseline prognostic factor is mental status at the time of presentation. Individuals with altered sensorium have a much worse prognosis than those who are awake and alert [27, 47, 49]. High fungal burden, a positive India ink test, elevated intracranial pressure, and lack of inflammatory cells in the CSF are also associated with poor outcomes [25, 27, 28, 47, 49, 50]. Early detection of cryptococcal infection with serum CRAG testing and treatment with fluconazole can decrease the rate of progression and mortality from cryptococcal disease in resource-limited settings [42].
Treatment
Early diagnosis is crucial to improving treatment outcomes for HIV-infected patients with cryptococcosis. Other key factors for providing optimal care include early induction therapy for meningoencephalitis followed by suppressive regimens, early recognition and management of elevated intracranial pressure and IRIS, and the use of lipid formulations of amphotericin B in patients with renal impairment [41]. However, in many settings, management options may be limited by the medical resources available. In all cases, treatment of the underlying immunosuppression from HIV infection is critical to managing cryptococcosis
The treatment of cryptococcal meningoencephalitis in AIDS patients requires initial induction therapy with a fungicidal regimen, preferably amphotericin B and flucytosine, followed by suppressive therapy with fluconazole (Table 29.3). Amphotericin B is more effective than azole therapy during the initial phase of treatment, leading to faster sterilization of the CSF and fewer relapses [47, 51, 52]. The addition of flucytosine increases the efficacy of amphotericin [28, 41, 52]. A randomized study from Thailand showed that the combination of amphotericin B and flucytosine cleared Cryptococcus from the CSF faster than amphotericin B alone, amphotericin B and fluconazole, or triple therapy with amphotericin B, flucytosine, and fluconazole [53]. The prospective multicenter study CryptoA/D also showed that flucytosine therapy was associated with mycologic clearance at 2 weeks of therapy [48]. Therefore, amphotericin and flucytosine combination therapy is recommended in the USA. Of note, flucytosine causes significant hematologic toxicity, and levels and hematologic paramenters should be monitored closely. Patients who develop amphotericin-related nephrotoxicity or infusion reactions can be treated with lipid formulations of amphotericin, which are associated with less toxicity and similar efficacy, but are more costly [54–56].
Table 29.3 Therapy of HIV-associated cryptococcal meningoencephalitis
| Regimen | Duration | Evidencea |
|---|---|---|
| Induction therapyb | ||
| Preferred | ||
| Amphotericin B (0.7–1.0 mg/kg/day)c + flucytosine (100 mg/day)d | 2 weeks | 1 |
| If flucytosine intolerant: | ||
| Amphotericin B (0.7–1.0 mg/kg/day) or liposomal amphotericin B (3–4 mg/kg/day or amphotericin B lipid complex (5 mg/kg/day) | 4–6 weeks | 2 |
| Alternatives for resource-limited settings | ||
| Amphotericin B (0.7-1.0 mg/kg/day) + fluconazole (800 mg/day) | 2 weeks | 1 |
| Fluconazole (>800 mg/day) + flucytosine | 6 weeks | 2 |
| Fluconazole (>1200 mg/day) | 10–12 weeks | 2 |
| Consolidation therapy | ||
| Fluconazole (400–800 mg/day)e | 8 weeks | 1 |
| Maintenance therapy | ||
| Fluconazole (200 mg/day) | Until CD4 count > 100 cells/mm3 and/or viral load undetectable for > 3 months with at least 1 year of total therapy | 1 |
| Primary prophylaxis | Not recommendedf | |
a Evidence 1 = randomized clinical study, 2 = clinical trial > 20 patients
b Manage elevated intracranial pressure with serial lumbar puncture if necessary.
c Alternative: liposomal amphotericin B (3–4 mg/kg/day) for patients with renal dysfunction.
d Monitor renal function on amphotericin, and flucytosine blood levels and/or hematologic parameters.
e Use higher-dose fluconazole (800 mg/day) during consolidation if using alternative induction regimen.
f Consider checking serum cryptococcal antigen in areas with high incidence of cryptococcemia prior to ART initiation.
In resource-limited settings, optimal induction therapy with amphotericin B and flucytosine may not be feasible due to lack of medications or inability to monitor toxicity, and oral fluconazole is an alternative for the treatment of cryptococcal meningoencephalitis in patients with mild symptoms and normal sensorium. Results from several studies evaluating the efficacy of fluconazole monotherapy for cryptococcal meningoencephalitis have been mixed [57, 58], but a recent study found treatment success rates over 60% using higher doses of fluconazole (1,600–2,000 mg daily) [59]. Nausea and vomiting are common, but may be alleviated by administering 3 to 4 divided doses daily. Therefore, patients with cryptococcal meningoencephalitis without neurologic complications may be appropriately treated with 10 weeks of oral high-dose (> 800 mg daily, 1,200 mg/day preferred) fluconazole followed by lower-dose maintenance therapy (200–400 mg daily) [41]. Primary and secondary fluconazole resistance may be an issue in patients treated with fluconazole alone, and MIC testing may be indicated in some cases. In a small retrospective study of HIV-infected African patients with cryptococcal meningoencephalitis treated with fluconazole monotherapy, 76% of the isolates recovered during treatment failure had reduced susceptibility to fluconazole [60]. If flucytosine is not available, fluconazole (800 mg/day) can be used as a substitute in combination with amphotericin B during the first two weeks of induction therapy [41].
Following the 2-week induction phase with parenteral therapy, patients can be switched to oral fluconazole (400 mg/day) for 8 weeks (consolidation therapy). It is commonly recommended that CSF be evaluated for sterility after this period. The cryptococcal CSF antigen may remain positive for up to 1 year after successful therapy, so fungal cultures of the CSF should be performed. Prolongation of induction therapy is appropriate in patients who remain comatose, have persistently elevated intracranial pressures, or are clinically deteriorating [41].
Persistent infection is generally defined as persistently positive CSF cultures after 4 weeks of adequately dosed therapy [41]. Due to a paucity of relevant trials, recommendations for “salvage therapy” are predominantly based on expert opinion. Patients with persistently positive cultures should have their immune status optimized with ART and induction therapy should be reinitiated, usually with higher doses of amphotericin. If possible, drug resistance should be evaluated in persistent isolates. Small open-label salvage trials have reported treatment success with voriconazole or posiconazole as salvage therapy in 40–50% of patients with persistent cryptococcosis [41, 61, 62].
Cerebral cryptococcomas
Cerebral cryptococcomas are rare but require prolonged courses of induction and maintenance therapy. Induction therapy with amphotericin B and flucytosine is recommended for at least 6 weeks, followed by consolidation therapy with fluconazole (400–800 mg daily) for 6 to 18 months, depending on clinical response. Corticosteroid therapy may be necessary in cases of mass effect surrounding the lesions, and surgery may be appropriate for large accessible lesions associated with mass effect [41].
Management of elevated intracranial pressure
Elevated intracranial pressure is a common and potentially life-threatening complication of cryptococcal meningoencephalitis [7, 63]. Early recognition and treatment of increased intracranial pressure is a key principle for optimal management [41]. In contrast to the usual pathophysiology of chronic meningoencephalitis, in which pro-inflammatory responses play an important role, cryptococcal meningoencephalitis in HIV-infected patients is notable for a lack of inflammatory cells and seems to be directly related to high fungal titers which cause an outflow obstruction. However, immune reconstitution in patients receiving ART may lead to an inflammatory response that increases intracranial pressure.
In a retrospective study of 211 HIV-infected patients with cryptococcal meningoencephalitis, 60% were found to have initial opening pressures of ≥ 250 mmH2O, and 30% had a pressure ≥ 350 mmH2O [49]. Those with opening pressures ≥ 350 mmH2O had a significantly higher fungal burden and were more likely to have papilledema, hearing loss, and meningismus [49]. Interestingly, this group was less likely to have fever and night sweats, perhaps reflecting an impaired ability to mount an inflammatory response. However, clinical findings were not sensitive or specific predictors of intracranial pressure elevation, and opening pressures should be measured in all patients with cryptococcal meningoencephalitis without contraindication to a lumbar puncture. If available, radiologic imaging of the brain is recommended prior to lumbar puncture in patients with focal neurologic signs or impaired mentation [63]. In resource-limited settings where imaging is not available, no adverse outcomes have occurred in patients without focal neurologic deficits undergoing lumbar puncture with adequate monitoring of opening and closing pressures [64].
The best intervention for elevated intracranial pressure associated with cryptococcal meningoencephalitis is drainage of the CSF. This can usually be accomplished by large-volume lumbar punctures removing up to 30 mL of CSF at one time, which should be repeated daily until the intracranial pressure normalizes and symptoms have been stabilized for at least 2 days [41]. Patients who require frequent lumbar punctures or whose opening pressure is > 400 mm H2O may benefit from a lumbar drain. Ventriculoperitoneal shunting may be indicated for persistently elevated pressures or progressive neurologic deficits [41, 65–68]. Patients with rising intracranial pressure while on treatment are at high risk of adverse outcomes and death [49].
Perhaps due to the sparse cellular infiltrate and limited host response, corticosteroids do not improve clinical outcomes in HIV-associated cryptococcal meningoencephalitis [49]. However, patients experiencing major complications from ART-associated IRIS may benefit from corticosteroids. A 2- to 4-week course of steroids is reasonable, but may be extended depending on the clinical scenario and patient response. There is not enough experience to recommend non-steroidal anti-inflammatory agents or thalidomide for the treatment of IRIS.
Relapses
Over one-third of patients successfully treated for cryptococcal meningoencephalitis will relapse without maintenance therapy [69]. Relapse rates are even higher in patients treated only with fluconazole during induction therapy [60]. Therefore, following the 8 weeks of 400 mg/day fluconazole, patients must be placed on suppressive antifungal therapy [69]. Lower-dose fluconazole (200 mg/day) is the preferred maintenance regimen. Fluconazole maintenance therapy is superior to amphotericin or itraconazole for preventing relapses [70]. Another major strategy to prevent relapses is the initiation of ART. Several studies in patients with a history of cryptococcal meningoencephalitis have shown that maintenance therapy can be safely discontinued in patients on ART with sustained immunologic and virologic responses (CD4 counts > 100 cells/mm3 or undetectable viral load for more than 3 months) [71–75]. In a retrospective multicenter study of 100 patients with a history of cryptococcal meningoencephalitis, the incidence of relapse after discontinuation of fluconazole was only 1.5/100 person-years among patients on ART with a CD4 counts > 100 cells/mm3 [73]. In a small trial of 42 patients with cryptococcal meningoencephalitis randomized to continue or discontinue fluconazole maintenance therapy when the CD4 count increased to greater than 100 cells/mm3 and the viral load was undetectable, there were no relapses in the discontinuation arm after 48 weeks of follow-up [75].
Timing of ART and IRIS
There are no large studies that definitively establish the best timing for ART initiation in patients with cryptococcal meningoencephalitis. In a retrospective study from France, 10 out of 120 patients with cryptococcal meningoencephalitis who initiated ART developed IRIS, and 3 of the 10 died. Development of IRIS was associated with initiating ART within 2 months of the diagnosis of cryptococcosis [37]. In contrast, a recent prospective cohort study of 101 AIDS patients with cryptococcal meningoencephalitis found an association between high cryptococcal antigen titers and the risk of IRIS, but not with the timing of ART [38]. In a study of 282 AIDS patients with opportunistic infections,early ART (within 14 days of starting opportunistic infections treatment) improved survival/AIDS progression compared to delayed ART (after completion of acute opportunistic infections therapy), and there was no association between timing of ART and IRIS [76, 77]. Although only 35 patients in this trial had cryptococcal meningoencephalitis, there was a trend towards improved survival in patients receiving early ART [77]. The most recent guidelines from the Infectious Disease Society of America recommend initiating ART within 2 to 10 weeks of diagnosis of cryptococcal meningoencephalitis [41]. Prolonged delays in ART initiation should be avoided to prevent other complications and mortality from untreated HIV infection. Screening for asymptomatic antigenemia should be considered in patients with CD4 counts < 100 cells/mm3 living in high incidence areas, such as sub-Saharan Africa or Southeast Asia. Subclinical antigenemia can successfully be treated with fluconazole, but without therapy is a major risk factor for IRIS [42, 78–80].
Extraneural Cryptococcosis
The incidence of extraneural cryptococcal disease in AIDS patients ranges between 20 and 60% [7, 26, 81, 82]. Despite entry through the lung, evidence of pulmonary symptoms is present in only 20–30% of cases. Other sites of extraneural involvement include the joints, oral cavity, pericardium, myocardium, skin, mediastinum, and genitourinary tract.
Diagnosis
The serum CRAG test has excellent sensitivity and specificity for the diagnosis of disseminated cryptococcosis without CNS involvement. Serum CRAF ≥ 1:8 should always be treated regardless of symptoms. Fungal blood cultures are often positive and bone marrow examination does not increase the diagnostic yield [83]. In contrast to immunocompetent patients with cryptococcal pneumonia, in whom the serum CRAG is often negative, HIV-infected patients with pulmonary cryptococcosis usually have a positive test [84]. HIV-infected patients with pneumonia and a CD4 count ≤ 100 cells/mm3 should also have fungal cultures of sputum performed. C. neoformans can be readily identified using methenamine silver, mucicarmine, and periodic acid-Schiff stains, but cannot be detected with regular Gram’s stain of tissue.
Treatment
In immunocompetent patients, asymptomatic pulmonary cryptococcosis often resolves spontaneously and does not require antifungal therapy. In HIV-infected patients, however, pulmonary cryptococcosis should always be treated to prevent disseminated disease [41]. Isolated cryptococcemia indicates deep tissue invasion and should also be treated. In any presentation of extraneural disease, a lumbar puncture should be performed to rule out CNS involvement.
The treatment of extraneural cryptococcosis does not usually require parenteral therapy except in cases of severe pneumonia (acute respiratory distress syndrome) which should be treated like CNS disease. Most cases of mild to moderate disease are treated with daily oral fluconazole (400 mg/day) [57]. The length of therapy has not been well established in clinical trials, though most experts advocate continuing lifelong therapy. In patients on ART, therapy may be discontinued after 1 year of treatment if the CD4 count rises over 100 cells/mm3 and the cryptococcal antigen titer is < 1:512 [41, 85].
Primary fungal prophylaxis
Three studies have evaluated the role of primary prophylactic antifungal therapy for HIV-infected patients with low CD4 counts [86–88]. Two studies conducted in the USA showed that fluconazole prophylaxis decreased the incidence of cryptococcosis, and itraconazole prophylaxis decreased the incidence of both cryptococcosis and histoplasmosis, especially in patients with CD4 counts < 50 cells/mm3, but there was no survival benefit [87, 88]. Given the relatively low prevalence of systemic fungal infections in the USA, the widespread use of ART, and the risk of promoting drug resistance, routine antifungal primary prophylaxis is not routinely advocated. However, it may be considered in HIV-infected patients with CD4 counts < 100 cells/mm3 in areas where there is limited availability of ART and the incidence of cryptococcosis or other fungal infections, such as histoplasmosis or coccidioidomycosis, is high. Asymptomatic cryptococcal antigenemia has been associated with increased mortality in patients initiating ART. Screening patients with CD4 counts < 100 cells/mm3 with CRAG testing and offering preemptive therapy for patients with a positive result is cost-effective in settings with high incidence of cryptococcosis [42, 89].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree