2 Consultant Paediatrician
3 Specialist Practitioner in Children’s Community Nursing, Nottingham Children’s Hospital
- complications with lung development
- common congenital anomalies of lungs
- the causes of bronchial wall destruction
- the significance of nursing support.
Chronic lung disease of prematurity
Chronic lung disease of prematurity (CLD) (synonyms: chronic neonatal lung disease (CNLD), chronic lung disease of infancy (CLDI), bronchopulmonary dysplasia (BPD)) was first described by Northway and colleagues more than 40 years ago and included clinical and radiological features in preterm infants who had severe respiratory distress syndrome (RDS), who had been treated with high inspired oxygen (O2) concentrations and prolonged mechanical ventilation with high positive airway pressures, resulting in inflammation, fibrosis and smooth muscle hypertrophy in the airways (‘classic or old BPD’) (Northway et al. 1967). Neonatal intensive care has advanced beyond recognition since this early description but despite advances in the prevention and management of RDS such as the use of antenatal steroids, surfactant treatment, better nutritional interventions, careful monitoring of oxygen therapy, better ventilators and ventilation strategies, CLD is still one of the major complications in mechanically ventilated neonates.
Any disorder that produces an acute lung injury and/or requires treatment with positive pressure mechanical ventilation and high concentrations of inspired oxygen during the initial weeks of life predisposes the infant to the development of CLD.
With the survival of extremely premature infants (23–24 weeks’ gestation), a different type of CLD has emerged. This type of CLD (‘new BPD’) represents a disorder of intrauterine inflammation and premature extrauterine lung development characterised by alveolar simplification, in contrast to the early descriptions of BPD, in which postnatal inflammation and fibrosis due to barotrauma and oxygen toxicity played more of a role (American Thoracic Society 2003).
Definition
Bancalari et al. (1979) maintain that there are three basic criteria required to define BPD:
- supplemental oxygen requirement at 28 days of postnatal life
- persistent abnormalities of the chest radiograph
- tachypnoea in the presence of crackles, which are the crackling noises heard on auscultation during inhalation.
However, some researchers feel that as ‘new BPD’ occurs in very low-birthweight infants with gestational ages of 30 weeks or less, the cut-off for supplemental oxygen should be 36 weeks postconceptional rather than beyond 28 days of age in ‘old or classic BPD’ (Shennan et al. 1988).
The BTS guidelines (2009) define CNLD as an infant requiring supplemental oxygen at a corrected age of 36 weeks’ gestation who is at least 28 days old.
It is difficult to estimate the true prevalence of BPD due to changing definitions but it is felt that as neonatal care has changed and more premature babies are surviving, the prevalence seems to have gone up but it is less severe now. CLD (oxygen requirement at 36 weeks after conception) developed in 19% of the very low-birthweight babies born during the period of 1993–1994 in an American study (Stevenson et al. 1998). In a more recent prospective follow-up study of extreme premature babies born at ≤25 weeks’ gestation in 1995 in the UK, 74% of this population received supplementary oxygen at 36 weeks’ postmenstrual age and 36% were discharged with supplementary oxygen, i.e. more than one-third of the babies born at ≤ 25 weeks’ gestation have CLD (Hennessy et al. 2008).
Pathophysiology
The cause of BPD is multifactorial. The immature lung is most vulnerable to disruption of alveolar development in the stage before alveolar formation begins (23–26 weeks’ gestation). Factors that increase inflammation in the lung – such as oxygen toxicity, mechanical ventilation-induced trauma from volume and pressure changes, and infection – are associated with the development of BPD. Exposure to inflammation of fetal membranes, such as chorion caused by bacterial infection, known as chorioamnionitis, with resultant foetal inflammatory syndrome and high levels of circulating proinflammatory cytokines, also places preterm infants at increased risk of BPD.
Foetal inflammatory syndrome, including high levels of circulating proinflammatory cytokines, which are inflammatory mediators, also places preterm infants at increased risk of BPD. Inflammation has an important role in the pathogenesis of BPD and the pharmacological modulation of the inflammatory response may be protective (Eichenwald and Stark 2007). The ‘new BPD’ is believed to represent less the effects of severe lung injury and its repair and more a disruption or arrest of lung development. Changes in the pulmonary vasculature structure (‘dysmorphic circulation’) are increasingly being recognised as contributory to the pathogenesis of CLD (Jobe and Bancalari 2001). A pulmonary score to define the severity of CLD has also been developed, which is useful to guide practice (Coalson 2000).
Treatment
Nutrition
Infants with CLD have higher energy needs compared to healthy age-matched infants. Some may have feeding difficulties secondary to oral aversion related to the lengthy neonatal intensive care unit (NICU) interventions and are more likely to have gastro-oesophageal reflux. This may also lead to difficulties with sucking and swallowing co-ordination due to evolving neurodevelopmental problems. It is important to optimise nutrition in these infants but this can be very challenging due to the above reasons. Feeding with calorie-dense formulae does result in ‘catch-up’ weight gain but height velocity often remains subnormal even up to school age (Madan et al. 2005).
Bronchodilators
The response in babies with CLD is variable and in symptomatic babies with definite wheeze, a trial of salbutamol or ipratropium via spacer may be given. If there is definite clinical improvement, these agents can be used on an as-required basis at times when the babies are symptomatic. An appropriate drug delivery device and facemask should be chosen.
Corticosteroids
Postnatal oral corticosteroids have been widely used in evolving or established CLD but their use still remains controversial. Early, moderately early and delayed use (Giacoia et al. 1997; Halliday et al. 2003a–c) are associated with significant side-effects including hyperglycaemia, hypertension, gastrointestinal bleeding, intestinal perforation, decreased growth, adrenal suppression, cardiomyopathy and interventricular septal hypertrophy and nosocomial infection. Delayed side-effects include abnormal neurological examinations, cerebral palsy and developmental delay. There are also concerns regarding decreased alveolar number (American Thoracic Society 2003).
In infants with severe BPD who are ventilator dependent, use of corticosteroids might improve survival without increasing adverse neurological outcome (Halliday et al. 2003a). More research is required to establish the optimal steroid as well as dosing regimen.
Inhaled corticosteroids (ICS) can facilitate extubation in ventilator-dependent infants when used over 1–4 weeks but the onset of action is slower compared to systemic steroids and no firm conclusions can be drawn about efficacy in non-ventilated infants (Doyle et al. 2005). Regular ICS reduce symptoms, improve lung function and lessen the need for bronchodilator therapy in those infants with CLD who are symptomatic at follow-up (Lister et al. 2000).
Oxygen therapy
This is covered in more detail in Chapter 6 but some of the aspects of oxygen therapy specific to babies with CLD are discussed in this section.
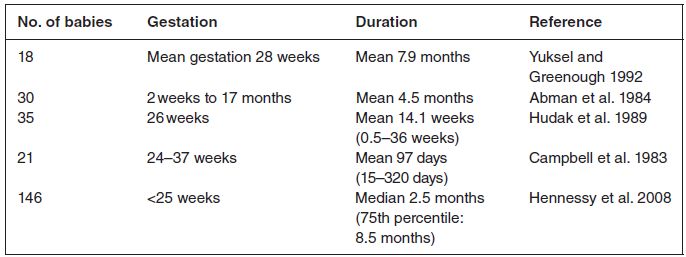
The duration of supplemental oxygen therapy is generally less than 6 months as shown in the studies listed in Table 11.1. In a child who is not weaning as expected from supplemental oxygen therapy, it is necessary to consider whether the child is not getting the oxygen because the supply has run out or if there is a problem with non-adherence. Other issues include unnoticed dislodgement of cannula and blocked tube or valve, all of which can be assessed by the nurse during home visits. This will be covered below.
Consequently, in CLD, failure to reduce oxygen supplementation after 1 year warrants specialist review to rule out other conditions such as:
- another or an additional medical diagnosis (unsuspected congenital cardiac defects, upper airway obstruction from enlarged tonsils and adenoids or subglottic cyst, and chronic aspiration with gastro-oesophageal reflux)
- condition increasing in severity, frequently after a respiratory illness
- pulmonary hypertension.
Very occasionally, long-term ventilation (LTV) at home may be required in infants with severe CLD who have never been able to be weaned from the ventilator in the NICU, or in those who have been weaned from the ventilator but have suffered a setback severe enough to warrant reinstitution of mechanical ventilation (American Thoracic Society 2003).
Table 11.1 Infants discharged home on supplemental oxygen
Diuretics
Despite the widespread use of different diuretics, little is known about the effects of long-term diuretic therapy in infants with developing or established CLDI with regard to survival, duration of ventilator support or oxygen administration, potential complications and long-term outcome (American Thoracic Society 2003).
Discharge planning
The importance of discharge planning cannot be overestimated. This involves prior preparation of the home environment, competent parents, equipment and the appropriate professional support. This will be discussed in more detail below.
Immunisation
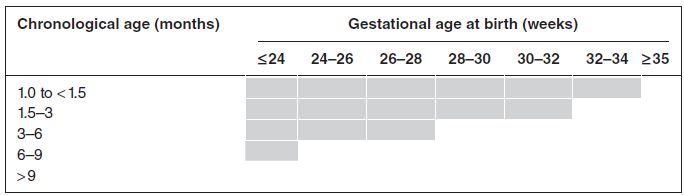
Passive immunisation with humanised monoclonal antibody (palivizumab) against the respiratory syncytial virus should be offered according to the Joint Committee on Vaccination and Immunization criteria. The JCVI considers palivizumab cost-effective when used as recommended for preterm infants with CLD (defined as oxygen dependency for at least 28 days from birth) at the chronological ages at the start of the RSV season and gestational ages at birth covered within the shaded area in Table 11.2.
Consider palivizumab for the prevention of RSV in the following categories:
- children with chronic lung disease (in other words, oxygen dependency for at least 28 days from birth) who have specific risk factors
- infants who have haemodynamically significant, acyanotic congenital heart disease and are less than 6 months old
- children who have severe combined immunodeficiency syndrome until they are immune reconstituted.
Other immunisations include influenza vaccine for the child after 6 months of age and for the close family contacts.
Table 11.2 Cost-effective use of palivizumab (shaded area) for preterm infants with CLD by chronological age (months) at the start of the RSV season (beginning of October) and gestational age at birth (weeks) (reproduced from Department of Health 2010)
Other issues
Parents and carers should be counselled about environmental tobacco smoke exposure, avoidance of viral exposures as far as possible and the importance of hand washing to prevent spread of infections.
Parents should also be counselled that infants with CLD are likely to require significant escalation of care including oxygen requirement and paediatric intensive care unit (PICU) care may be required in some infants, especially in the winter season.
Long-term complications can include lung transplantation. This may be indicated, although very rarely, in a small number of infants with very severe CLD when all other treatment has failed.
Modern medicine has assisted in the process of better clinical outcome and prognosis for infants with CLD. With companies providing home oxygen and children’s community nursing support as discussed in Chapter 3, children with this congenital abnormality of the lungs can be cared for in their homes successfully with the ultimate aim of weaning from oxygen therapy.
Interstitial lung disease
The pulmonary interstitium is the tissue in between the air sacs (alveoli) and the capillaries. Gas exchange occurs by diffusion across the epithelium of the capillaries across the interstitial space, and across the alveolar epithelium.
Definition
Interstitial lung disease(s) (ILD) are a heterogeneous group of rare disorders of known and unknown aetiology, characterised by derangement of alveolar walls with resultant impairment of gas exchange and diffuse infiltrates on imaging (Fan et al. 2004). The clinical presentation includes chronic tachypnoea, hypoxia, cough and/or crackles.
Sometimes the acronym chILD (childhood interstitial lung disease) is used to describe a group of disorders which have similar presentation though the severity of these diseases and long-term outcomes may be very different. ILD and diffuse paediatric lung disease have also been used synonymously but subsequent lung biopsy often reveals that the pathogenesis of many of these disorders is outside the interstitial compartment in the lungs, often with airway and airspace involvement (Deutsch et al. 2007).
The prevalence rate of idiopathic ILD in UK and Ireland for children aged 0–16 years is estimated to be 3.6 cases/million (Dinwiddie et al. 2002). Conditions included under the umbrella term of chronic ILD, as identified by Clement (2004) include pulmonary alveolar proteinosis which is a rare condition with abnormal accumulation of surfactant occurring within the alveoli, affecting gas exchange; hypersensitivity pneumonitis arises as a result of inflammation of the alveoli caused by hypersensitivity to inhaled dusts. Another is Langerhans cell histiocytosis, a rare disease involving growth of abnormal Langerhans cells, deriving from bone marrow, which can affect organs in the body such as the lungs. Finally, sarcoidosis is a disease where the cause is unknown and results in inflammation, affecting various organs in the body.
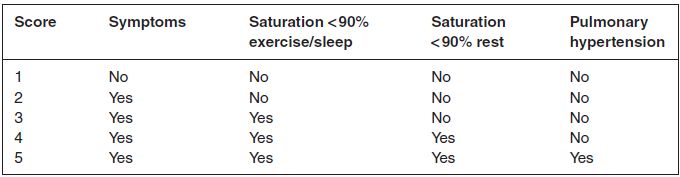
Table 11.3 Severity of illness score (adapted from Fan et al. 2004)
These conditions are much rarer compared to ILD in adults and at least in children under 2 years of age, the classification system used in adults cannot be applied as the aetiology in some cases, rate of progression and treatment approaches are different and some conditions are unique to early childhood, for example neuroendocrine cell hyperplasia and pulmonary interstitial glycogenosis (Deutsch et al. 2007).
Clinical presentation
Fan et al. (2004) and Clement (2004) state that the onset is often insidious and symptoms may have been present for months to years before the diagnosis of ILD is confirmed. The presentation could be of asymptomatic with radiological features suggestive of ILD. In others, symptoms at presentation include cough which is often dry and tickly, dyspnoea, tachypnoea and chest recession, exercise limitation, tiring during feeding, frequent respiratory infections, haemoptysis and failure to thrive. Parent/carer-reported wheeze is confirmed in some patients on examination but more often inspiratory crackles, tachypnoea and retraction are present.
It is recommended that any child with a normal birth history presenting with the signs and symptoms suggestive of ILD lasting for >3 months should be evaluated for ILD (Table 11.3). Clinical history should include details of relatives or siblings with similar lung conditions, possible precipitating factors, such as feeding history, any acute or severe respiratory infections, environmental exposure to organic and inorganic dust, and use of drugs with pulmonary toxicity.
Diagnosis
Investigations to identify primary disorders that predispose to ILD include:
- immune studies: HIV, immunoglobulins including IgE, skin tests for delayed hypersensitivity, response to immunisations, T- and B-cells
- others as indicated: barium swallow, pH probe
- infectious disease evaluation (cultures, titres, skin tests)
- genetic studies for surfactant dysfunction, serum and urine amino acids.
Pulmonary function tests
The picture may be one of mixed restrictive/obstructive disease. Most patients with mild disease have normal oxygen saturation levels. As the disease progresses or from the outset in severe cases, the patients may desaturate with exercise or during sleep due to ventilation/perfusion mismatch as discussed in Chapter 6. Patients with more advanced disease will be hypoxaemic at rest. Some patients develop pulmonary hypertension and this carries a poor prognosis. Electrocardiogram and echocardiogram will be required to assess development of pulmonary hypertension.
High-resolution computed tomography
This provides information about the extent and distribution of disease and is also helpful in determining the site of lung biopsy. High-resolution computed tomography (HRCT) may demonstrate geographic hyperlucency, where one lung is less dense than the other normal lung, cysts and nodules, and consolidation (Lynch et al. 1999). In infants, sedation is required and the child is hyperventilated by applying positive pressure through a facemask to produce a brief respiratory pause. This will result in motionless HRCT images obtained at either full inflation or resting end-expiration (Long et al. 1999).
Bronchoscopy and bronchoalveolar lavage
These are discussed in more detail in Chapter 4. Bronchoscopy will help to establish opportunistic infection (immunocompromised host) and other abnormalities such as alveolar haemorrhage of any cause including idiopathic pulmonary haemosiderosis (IPH), alveolar proteinosis and surfactant protein deficiency in children.
Lung biopsy
This is performed as either an open or thoracoscopic procedure to provide a tissue diagnosis.
Treatment
Fan et al. (2004) suggest that treatment should be based around the following factors.
Oxygen
To treat hypoxaemia.
Nutrition
Should be optimised as poor growth (weight) is an adverse prognostic factor.
Corticosteroids
Certain aspects of chILD will respond very well to corticosteroids, including hypersensitivity pneumonitis. Other conditions thought to be steroid responsive include chILD associated with connective tissue disease. Steroids are preferably given as intravenous pulse therapy rather than oral daily or alternate-day therapy because it is associated with fewer side-effects. The recommended dose of methylprednisolone is 30 mg/kg with a maximum of 1 g, given intravenously over 1 h, daily, for 3 consecutive days and repeated monthly.
Steroid-sparing agents
These include hydroxychloroquine, azathioprine, cyclophosphamide, methotrexate, ciclosporin, and intravenous gammaglobulin, and will be required in some children with ongoing active disease despite steroid therapy or if they develop unacceptable side-effects with corticosteroids.
Lung transplantation
More children are receiving lung transplantation for end-stage chILD and the survival rates are at least as good for chILD as they are for cystic fibrosis and pulmonary hypertension (for further details see Chapter 13).
Outcome
The prognosis for children with ILD is variable (Fan et al. 2004). Some of these conditions have a better prognosis, such as pulmonary interstitial glycogenosis; these children generally do well, although they may remain symptomatic and require oxygen for years. At the other end of the spectrum, neonates and infants do poorly, as well as older children with ILD and growth failure, pulmonary hypertension and severe fibrosis.
Children with ILD have a better outlook with the advancement of technology, including drug therapy. Although many do require oxygen therapy for a considerable time, support and management to facilitate quality of life from a multidisciplinary perspective are paramount.
Bronchiectasis
Bronchiectasis is defined as dilation of the airways accompanied by inflammatory destruction of the bronchial and peribronchial tissue. Developed countries have seen a decline in infection-related bronchiectasis with the use of antibiotics and implementation of vaccination programmes. However, it still causes significant morbidity in the paediatric population. Children present with chronic productive cough and recurrent chest infections.
Rene Laennac, inventor of the stethoscope, first described bronchiectasis in the early 19th century in patients with tuberculosis and the sequelae of pneumonia in the preantibiotic era. The term bronchiectasis is derived from Greek word bronchion, meaning windpipe, and ektasis, meaning stretched.
Bronchiectasis is frequently divided into cystic fibrosis (CF) and non-cystic fibrosis types. This section mainly focuses on non-CF bronchiectasis.
Pathogenesis
The pathogenesis is thought to be bronchial obstruction with retention of secretions and infection, ultimately causing bronchial wall destruction. In bronchiectasis there is impairment of the mucociliary function, leading to increased accumulation of secretions which become more viscous, exposing the patient to bacterial infections. Recurrent bacterial colonisation leads to progressive airway injury. There is infiltration with inflammatory mediators, which are molecules that act at the site of infection, for example neutrophils, T-lymphocytes, interleukins, elastase and collagenase, which leads to destruction of elastic and muscular components of the bronchial wall (Sepper et al. 1995). The part of the bronchi affected may become cylindrical, tubular or saccular. Bronchiectasis can present as either focal or diffuse disease involving both lungs. The diameter of the bronchi becomes bigger than the adjacent pulmonary artery which is a characteristic sign seen on HRCT scan.
Viral: adenovirus, influenza, respiratory syncytial virus
Mycobacterium: tuberculosis
Fungal: aspergillosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


