Chapter 14 Complications resulting from antiretroviral therapy for HIV infection
Introduction
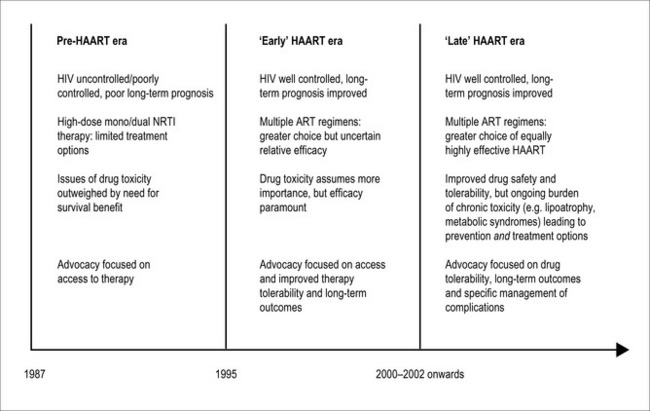
The treatment of HIV infection has passed through a number of distinct phases over the three decades of the HIV/AIDS pandemic, accompanied by significant shifts in both patients’ and clinicians’ perceptions of antiretroviral drug toxicity (Fig. 14.1). Prior to the introduction of ‘highly active antiretroviral therapy’ (HAART) regimens around 1996, any concerns regarding long-term drug toxicities were dramatically outweighed by the obvious short-term benefits and improved survival associated with these combination drug regimens. These benefits were soon realized, and the dramatic reduction in AIDS-related deaths following the introduction of HAART regimens marks a turning point in the progression of the HIV pandemic. At the same time, however, the ‘early HAART era’ from 1996 to around 2002 was characterized by the emergence of antiretroviral therapy (ART)-associated adverse events, which became an important source of morbidity and even mortality, threatening to outweigh AIDS-related events in frequency and overall detrimental effects on quality of life even in advanced HIV disease [1]. With evidence that numerous ART regimens have comparable efficacy in the treatment of HIV infection, the choice of therapy is now largely determined by the differential tolerability and risk of drug toxicity associated with individual HIV drugs.
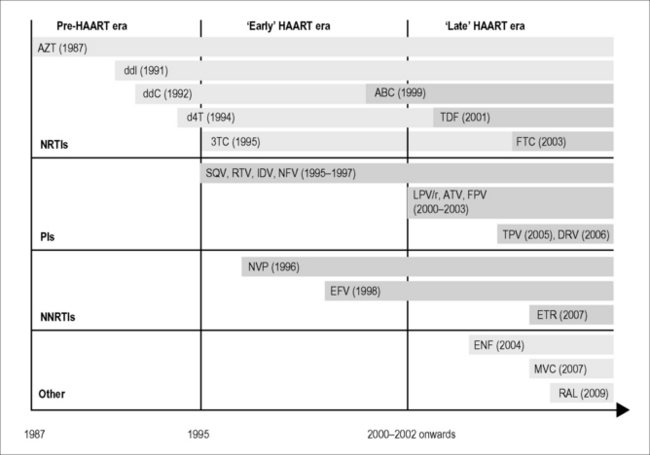
We have now moved into a ‘late HAART era’, characterized by the availability of newer-generation antiretroviral drugs that combine durable potency with improved safety profiles (Fig. 14.2), in which the realistic prospect of survival with HIV infection into old age has increasingly focused our attention on the prevention and management of prevalent diseases among those who have survived with HIV infection for many years. This new era has also seen the introduction of new classes of antiretroviral drugs, most notably integrase inhibitors (e.g. raltegravir) and CCR5 inhibitors (e.g. maraviroc), which thus far have proven to be safe and effective additions to the already wide range of available treatment options. Clinical experience with these newer agents is still relatively limited, however, and vigilance will need to be maintained to ensure that adverse events (for example, isolated case reports of rhabdomyolysis [2] and CNS toxicity [3] associated with raltegravir treatment) are reported and further characterized where possible. It is also apparent that while the incidence of adverse HIV drug reactions has decreased [4], previous drug toxicities involving end-organ damage (for example, neuropathy and lipoatrophy) can provide an ongoing burden of disease that may in turn contribute to an increased risk of diseases associated with aging. In this context, there is an ongoing need not only to prevent these complications whenever possible but also to develop effective treatment strategies for those affected by these complications of HIV therapy.
Adverse Effects of Antiretroviral Treatment in the Early Phase of Therapy
Mild cutaneous drug reactions can occur during the initial weeks of treatment, most notably associated with non-nucleoside reverse transcriptase inhibitors (NNRTIs; nevirapine, efavirenz, etravirine), protease inhibitors (PIs, especially fosamprenavir and darunavir), and less commonly with nucleoside analog reverse transcriptase inhibitors (NRTIs; tenofovir, or abacavir in the setting of the abacavir hypersensitivity reaction). These reactions are usually maculopapular in appearance, are generally non-progressive, and may not require cessation of therapy. However, assessment for the presence of mucosal involvement, fever, or signs of systemic involvement should be undertaken in order to exclude less common cases of more severe cutaneous drug reactions or systemic drug hypersensitivity reactions [5]. These more severe drug hypersensitivity reactions occur most commonly with abacavir or nevirapine therapy, although in both cases there have been significant advances in understanding the pathogenesis of these syndromes, which in turn have had a dramatic impact on clinical management.
Abacavir hypersensitivity reactions
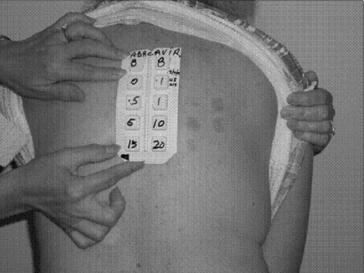
Abacavir hypersensitivity reaction (HSR) was the most frequent adverse event associated with use of this drug, consistently reported in ~8% of predominantly Caucasian abacavir-exposed individuals in the pre-marketing phase of development. Fortunately, more recent, widespread genetic screening for HLA-B*5701, an allele strongly associated with abacavir hypersensitivity, has led to elimination of immunologically confirmed hypersensitivity. Abacavir HSR is commonly characterized by constitutional and gastrointestinal symptoms (fever, malaise, lethargy, vomiting, abdominal pain, diarrhea), with possible respiratory involvement and skin exanthema as a possible late manifestation. Symptoms associated with abacavir HSR overlap with many other clinical syndromes associated with HIV and ART, such as immune restoration inflammatory syndromes (IRIS), opportunistic diseases, and other drug hypersensitivity reactions (e.g. associated with NNRTIs or trimethoprim-sulfamethoxazole), which led to overdiagnosis and false-positive clinical diagnosis of abacavir hypersensitivity. Abacavir patch testing has been used as a research tool in clinical studies to overcome this problem and by identifying true immunologically mediated abacavir HSR (Fig. 14.3) [6].
The large PREDICT-1 clinical trial, as well as numerous subsequent population-based studies, have demonstrated that HLA-B*5701 screening eliminates abacavir HSR confirmed by patch testing [7], in keeping with studies of the immunological basis of this reaction [8]. The SHAPE study further demonstrated a 100% negative predictive value generalizes in both black and white patients [9]. Prospective genetic testing for HLA-B*5701, therefore, effectively prevents at-risk individuals being exposed to abacavir. It is an excellent example of cost-effective, personalized genetic medicine that has been incorporated into international HIV guidelines and widely implemented in primary practice [10].
Nevirapine hypersensitivity reactions
Adverse drug reactions associated with nevirapine lead to discontinuation of this treatment in ~6–7% of treated individuals, and unfortunately, rare fatal cases associated with fulminant liver injury and/or severe cutaneous eruptions such as toxic epidermal necrolysis continue to be reported [11]. Nevirapine safety data compiled prior to 2003 demonstrated that severe (grade 3–4) hepatotoxicity and rash occur during the early phase of treatment (within 12 weeks of treatment initiation) in ~5% of nevirapine recipients, and that low CD4 counts are relatively protective against the development of these severe toxicity syndromes [12]. These findings were subsequently reflected in the Federal Drug Administration (FDA) and European Medicines Agency (EMA) recommendations that nevirapine not be initiated in women with CD4 counts >250 cells/mm3 or in men with CD4 counts >400 cells/mm3 unless the benefit outweighs the risk. Subsequent studies have shown that the risk of nevirapine HSR among treatment-experienced patients with undetectable plasma HIV RNA levels (i.e. <400 copies/mL) prior to commencing nevirapine treatment is comparable to that of “low risk” treatment-naïve patients [11]. Conversely, studies in resource-limited settings where nevirapine is used as first-line therapy have not consistently confirmed an increased risk of adverse drug reactions (rash or hepatic injury) associated with baseline CD4 counts [13].
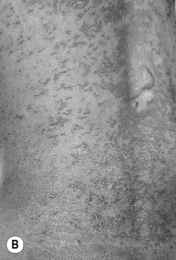
Nevirapine HSR appears to be relatively heterogeneous in nature, with variable expression of the component features of rash (Fig. 14.4), fever and constitutional illness, peripheral eosinophilia, and hepatotoxicity. It is particularly noteworthy that severe hepatic injury can occur in the absence of other clinical features. With regard to genetic risk factors, modest predictive values for nevirapine hypersensitivity have been associated with HLA-DRB1*0101 (hepatotoxicity, fever, and/or rash in Caucasians with CD4 >25 percent) [14] and HLA-B*3505 (rash alone in Asian (Thai) patients) [15].
Other potential pharmacogenetic strategies for preventing early treatment discontinuation
Nevertheless, these side effects can persist in some patients, and although these symptoms are not generally severe, they are a relatively frequent cause for treatment modification. This has in fact become a fruitful area of pharmacogenetic investigation in recent times, with several robust genetic associations suggesting that the tolerability of several HIV medications may have a strong genetic component [16]. For example, relatively frequent (>10%) genetic variants associated with Gilbert syndrome (especially UDP-glucuronosyltransferase (UGT)1A1 polymorphism) are associated with high rates of early atazanavir discontinuation (62 versus 15%); while genetic variants that influence efavirenz metabolism are also associated with early discontinuation (71 versus 20%) [16]. No genetic risk factors for tenofovir or lopinavir discontinuation were identified in this study.
Adverse Effects of Antiretroviral Treatment during Long-Term Therapy
Complications of nucleoside and nucleotide reverse transcriptase inhibitor (NRTI) therapy
Tenofovir renal safety
The renal safety of tenofovir has been a topic of interest since early reports (involving >30 cases) of significant renal toxicity with renal tubular damage and/or acute renal failure, with evidence of distinct renal pathological changes characterized by prominent proximal tubular injury [17]. In the large Gilead 903 study, in which participants had normal renal function at baseline, no evidence of significant renal toxicity could be identified over a 3-year treatment period using serum creatinine measures [18]. However, subsequent studies analyzed in a recent meta-analysis have demonstrated reproducible but modest reductions in glomerular filtration rate (of approximately 4 mL/min), with no discernible influence of tenofovir therapy on rates of acute or chronic renal injury or chronic renal failure, or of heavy proteinuria (>2 g/day), at the population level [19].
The overall prevalence of renal impairment or significant proteinuria in tenofovir-treated patients appears to have remained low at approximately 3% in both HIV-infected [20] and hepatitis B virus (HBV)-infected [21] populations, and attempts to identify useful clinical risk factors have not proved conclusive. Pre-existing renal impairment is known to be associated with risk of acute renal injury associated with tenofovir, and there is some evidence that alternative or additional explanations for renal impairment are frequent among tenofovir-treated patients with declining renal function [22]. However, current or previous renal impairment does not necessarily preclude the use of tenofovir in patients with limited treatment options [23], as long as renal function is appropriately monitored and proper dose adjustment is employed.
Lactic acidosis, hyperlactatemia, and acute hepatic steatosis
Lactic acidosis is probably the most severe clinical manifestation of mitochondrial dysfunction, in which loss of mitochondrial oxidative function leads to increased reliance on “anaerobic” metabolism and the inevitable accumulation of lactate. In the setting of NRTI therapy, there is now an appreciation of a spectrum of clinical disease associated with elevated systemic lactate levels [24]. At one end of this spectrum is a relatively common syndrome of mild, asymptomatic, nonprogressive hyperlactatemia (generally >2.5 mmol/L), which appears to represent a “compensated” homeostatic system in which elevated lactate production is balanced by effective mechanisms of lactate clearance. While the degree of hyperlactatemia appears to be greater in the presence of stavudine or didanosine therapy than of zidovudine or abacavir, this syndrome appears to be benign irrespective of the choice of NRTI therapy.
It is unfortunate to note that while this treatment complication has become exceedingly rare in developed countries with limited use of stavudine (or zidovudine), there are increasing reports of severe and fatal hyperlactatemia syndromes from resource-limited settings where stavudine is still frequently prescribed [25]. It is hoped that the implementation of updated treatment guidelines, along with increased recognition of the high costs associated with managing this and other drug toxicity syndromes associated with these thymidine analogue NRTI drugs [26], will soon change this situation.
Pancreatitis
The most comprehensive data concerning risk factors for NRTI-associated pancreatitis comes from the Johns Hopkins AIDS Service cohort (n = 2,613 cases) [27], with supporting data from the ACTG 5025 study. In these analyses, didanosine and stavudine were associated with roughly equivalent risk of pancreatitis, with estimated incidence rates of 0.8 and 1.1 cases per 100 person-years, respectively. Combining these drugs increased the risk approximately twofold, while concurrent didanosine and hydroxyurea use increased the relative risk approximately eightfold, including several fatal cases. More recently, cases of pancreatitis have been reported with concurrent use of didanosine and tenofovir, including with reduced doses of didanosine (250 mg/day), presumably due to the ability of tenofovir to “boost” didanosine effects in vivo [28].
Non-cirrhotic portal hypertension
A relatively rare syndrome (estimated prevalence ~8/10,000 patient-years) [29] of progressive portal hypertension associated with the development of ascites and varices has been noted among antiretroviral-treated patients. This syndrome is characterized by exuberant nodular regenerative hyperplasia and microvascular portal venopathy pathologically, and has been associated with didanosine exposure (odds ratio ~2), either alone or in combination with stavudine [29, 30], although cases have been described in the absence of exposure to these NRTIs [29]. Given the prominent role of venous thrombosis in the hepatic pathology, it is also proposed that additional factors such as enhanced microbial translocation from the gut and prothrombotic conditions may contribute to disease predisposition [31].
Neuropathy
The prevalence and long-term clinical impact of neuropathy is likely to be underestimated, as the symptoms are of gradual onset, and clinical signs of nerve damage are not always assessed in clinical practice. There is a definite contribution of HIV disease per se to the pathogenesis of a form of distal sensory neuropathy that is clinically and electrophysiologically indistinguishable from so-called “toxic neuropathy” from antiretroviral drugs [32]. It is therefore difficult to determine the relative contributions of disease- and drug-associated factors in the syndrome, as these effects are likely to be synergistic. The clinical syndrome common to both HIV-associated and toxic sensory polyneuropathy is dominated by peripheral pain and dysesthesia, with rare motor involvement.
In an international cohort study in which the overall prevalence of symptomatic peripheral neuropathy was approximately 50%, exposure to stavudine (odds ratio, OR 7.7) or didanosine (OR 3.2) were dominant risk factors, along with age >40 years (OR 2.9) [33].
Reversal of established neuropathy appears to be a slow process that is dependent on cessation of the offending NRTI, and there is limited evidence for treatment strategies other than topical capsaicin 8% [34], although several other agents such as gabapentin and tricyclic antidepressants have been used. As with any clinical neuropathy, a search for contributing factors such as diabetes, excessive alcohol consumption, and vitamin deficiencies (e.g. thiamine, B12, and folate) should be undertaken in patients who have neuropathy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree