Chapter 41 Complications of the newborn
Learning outcomes for this chapter are:
1. To outline the common causes of preterm births, and the characteristics of the neonate, along with problems faced by preterm neonates, and their management
2. To explain the normal physiology of bilirubin metabolism, identify causes of hyperbilirubinaemia in the preterm neonate and describe the assessment and management of jaundice of prematurity and pathological jaundice
3. To explain the aetiology for small for gestational age and intrauterine growth restriction
4. To outline the management for large-for-gestational-age infants
5. To describe the nutritional requirements and management for the preterm and sick neonate
6. To explain the neonatal immune system and its role, and discuss common neonatal infections
7. To identify the common causes of bleeding in the neonate
8. To explain the aetiology and risk factors for congenital abnormalities, and identify some common congenital abnormalities
9. To describe the most common types of cardiac lesions and their prognosis
10. To discuss some common neonatal respiratory disorders and various respiratory support measures
11. To describe the features of common neonatal metabolic and endocrine disorders
12. To identify the risk factors for sudden unexpected death in infancy (SUDI), and discuss provision of information to parents and families on reducing risk
13. To identify the clinical presentations of neonatal seizures
14. To discuss the associated risk factors with substance abuse during pregnancy.
congenital dislocation of the hip
Group B haemolytic Streptococcus
intrauterine growth restriction (IUGR)
small for gestational age (SGA)
sudden unexpected death in infancy (SUDI)
transient tachypnoea of the newborn
This chapter outlines the common complications of the newborn; it includes preterm birth, small and large for gestational age, jaundice, infections, neonatal seizures, and bleeding in the neonate. In addition, this chapter discusses congenital, cardiorespiratory, metabolic and endocrine disorders that could affect the neonate, neonatal abstinence and fetal alcohol syndrome, and sudden unexpected death in infancy (SUDI). It is not within the scope of this chapter to discuss all the neonatal disorders in detail—a brief outline of conditions is given. It is important to remember that, when caring for neonates, midwives must always be prepared for the unexpected in this fragile population.
THE LOW BIRTHWEIGHT NEONATE
Preterm neonate
Definition and causes
Preterm births account for approximately 10% of all births. Of all the Australian live births in 2006, 8.2% were between 32 and 36 weeks gestation; 0.9% were between 20 and 27 weeks, 0.8% were at 28–31 weeks and 65% were at 32–36 weeks (Laws & Hilder 2008). In the same period there were 13.7% preterm neonates born to Australian Indigenous and Torres Strait Islander mothers. This is greater than the rate of preterm births among non-Indigenous mothers (5.1%) (Laws & Hilder 2008).
The incidence of preterm births varies between and within countries, according to race and ethnicity, demographics, socioeconomics and environmental factors. Over the last 20 to 30 years the incidence of preterm births has increased by approximately 5%–7% of live births in most developed countries. The United States continues to have the highest incidence at 12% (Goldenberg & Culhane 2007; Tucker & McGuire 2005). This increase is thought to have been caused by an increase in induced preterm births, multiple births and advances in assisted reproductive technologies (Goldenberg & Culhane 2007; Tucker & McGuire 2005). In Australia, the incidence of spontaneous preterm births has risen by 20% since 1994 (ACM 2007), while in New Zealand the rate of singleton preterm births rose by 37.2% over a 20-year period from 1980 to 1999 (Craig et al 2002).
Preterm births can occur from either an induced or a spontaneous preterm birth. Induced preterm births account for 25% of all preterm births and are initiated by maternal or fetal indications; the rest are a result of spontaneous preterm births (Goldenberg & Culhane 2007). The cause of spontaneous preterm births is unknown in 40% of cases; however, there are certain predisposing antenatal factors, including:
• smoking, substance abuse or alcoholism in pregnancy
• overdistension of the uterus, for example multiple pregnancies, polyhydramnios
• fetal development abnormalities
• maternal age of <20 years or >35 years
• acute or chronic maternal diseases—such as pyelonephritis, chronic nephritis, essential hypertension
• primiparity (Henderson & Macdonald 2004; Johnston et al 2003).
According to the Perinatal Society of Australia and New Zealand Perinatal Death Classification, spontaneous preterm birth, alongside congenital abnormalities and unexplained antepartum deaths, accounts for over half of perinatal deaths (AIWH 2006). It has been well documented that preterm births are the primary cause of neonatal morbidity and mortality. Table 41.1 indicates the births by gestational age and birth status for 2006 in Australia.
Table 41.1 Neonatal births and deaths by gestational age, Australia 2006
| Gestational age (weeks) | Live births (%) | Fetal deaths (%) |
|---|---|---|
| 20–27 | 0.5 | 58.8 |
| 28–31 | 0.8 | 9.0 |
| 32–36 | 6.4 | 14.1 |
(Data from AIWH 2006)
These cut-off weights are based on global epidemiological observations whereby two-thirds of preterm babies are classified as low birthweight (Tucker & McQuire 2005). In Australia, approximately 2500 neonates are born each year weighing less than 1500 g, and over 1000 are born in the extreme low birthweight category (Monash Institute of Medical Research 2007). Very-low-birthweight neonates represent 3% of all live births in New Zealand. Of these, more than 37% are born at less than 32 weeks gestation and 32% weigh less than 1500 g (Australian Medical Association 2001).
Preterm neonates represent approximately 75% of neonatal intensive care unit (NICU) level III admissions. In 2004, 5724 Australian babies were admitted to an NICU, with 43.6% being of a gestational age of less than 32 weeks and 39.3% having a birthweight of less than 1500 g (AIWH 2006). Over the past decade, advances in neonatal intensive care have increased the limit of human viability to a much younger gestational age, although the survival rate for neonates born between 22 and 24 weeks is still very low (Pignotti & Donzelli 2008). Overall survival for preterm neonates at more than 28 weeks gestation is between 90% and 98% (Engle et al 2005).
Despite the increased survival of high-risk neonates this has not, however, resulted in decreasing the associated morbidity (American Academy of Pediatrics 2008). The majority of neonates born at less than 25 weeks gestation have an increased risk of death before, during or after
birth in the NICU. Those who survive are at risk of death during childhood, and approximately half will suffer from moderate or severe neurodevelopmental disability. At the age of six years, some of those previously considered to be healthy will show some kind of disability (MacDonald 2002; Pignotti & Donzelli 2008). Current evidence indicates that 90% of neonates born at 26–28 weeks gestation will develop into normal, healthy children (Johnston et al 2003).
Characteristics
• birthweight less than 2500 g
• thin, shiny, pinkish red skin
• little scalp hair, with lots of fine hair (lanugo) on the face and trunk
In addition, the preterm baby will have a head circumference that exceeds that of the chest. The chest tends to be relatively small and narrow, with no breast tissue. The face of the preterm neonate is triangular, with a pointed chin, and the fontanelles and sutures are widely spaced due to poor ossification. Ears on the preterm are easily folded, lacking firmed cartilage. Also, the length of the trunk in proportion to the limbs is greater in the preterm than in the term neonate. The skin in the preterm is pinkish red (plethoric) with prominent surface veins due to the absence of subcutaneous fat and increased level of red blood cells. The protective creamy white substance known as vernix is more plentiful. The limbs are thin, with decreased muscle tone, and the nails are soft. With neonates of less than 34 weeks gestation, the creases in the feet are almost absent. In the preterm female, the labia minora are prominent and gaping, and the male usually has incompletely descended testes. Furthermore the suck-and-swallow reflex is uncoordinated in preterm neonates of less than 34 weeks gestation.
Gestational age assessment
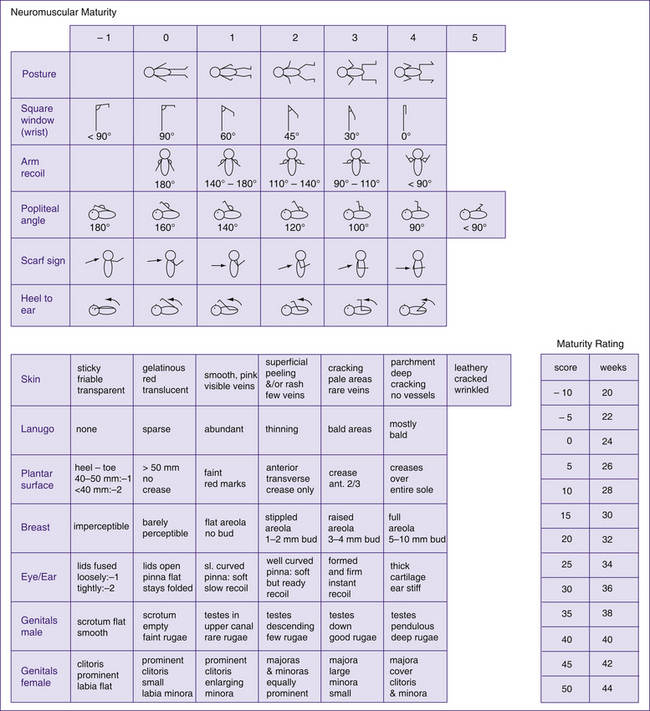
Many scoring systems have been developed over the years in order to estimate the gestational age of the neonate. These scoring systems require a complete and careful examination of the neonate. The scoring system examines developmental characteristics that are primarily related to the neonates’ maturity, thus providing the healthcare professional with an indication of whether the neonate is small for gestational age or preterm. Gestational assessment should be performed within the first 2–42 hours of life, as neuromuscular maturity can be influenced by maternal medications administered during labour. The two most commonly used scoring systems are the Dubowitz and, more recently, the Ballard, an adaptation of the former. The Ballard scoring system evaluates six physical and six neuromuscular characteristics of maturity. Each category is rated from a scale of –1 to 5 (–1 for extreme prematurity through to 5 for postmaturity). At the end of the assessment the physical and neuromuscular scores are added together and matched to the assessment tool (Fig 41.2, overleaf) to give an estimated gestational age.
Preterm labour and birth care
In most cases it should be possible to diagnose the onset of preterm labour early enough to ensure that the birth occurs in a maternity unit containing the necessary neonatal services and thus avoid the greater hazard of transfer after birth. During a preterm labour, the midwife should continuously monitor the fetal heart rate to detect any form of fetal compromise. The midwife should also avoid giving narcotics to the woman, in order to prevent respiratory depression in the neonate at birth. All resuscitation equipment must be checked and ready before the birth. It is important that an experienced neonatologist and midwife be present at the birth of the preterm to ensure immediate and expert resuscitation. The degree of resuscitative measures is relatively related to the gestation of the preterm neonate. In general, the approach taken to resuscitate neonates born at greater than 32 weeks gestation is the same as term neonates. Neonates born at less than 32 weeks gestation or less than 1500 g require more active support; while neonates of less than 28 weeks require intubation and assisted ventilation (Fowlie & McGuire 2005).
Cold stress at birth must be prevented, as this increases neonatal oxygen requirements and reduces surfactant production, resulting in hypoglycaemia and acidaemia (Fowlie & McGuire 2005). The neonate, therefore, must be dried quickly and thoroughly with warm towels and placed under a radiant heater immediately after birth.
Specific preterm problems
• Birth asphyxia—can occur if there is any disruption to the oxygen supply prior to or during birth, due to reduced energy reserves of the preterm neonate.
• Respiratory distress—this is the most common problem experienced by preterm neonates, and is exacerbated by younger gestational age. Preterm neonates have weak muscles and immature respiratory control. It is not uncommon for the preterm neonate to breathe well initially but later develop expiratory grunt, apnoeic episodes and cyanosis, requiring respiratory support. Respiratory conditions experienced by preterm neonates are discussed later in this chapter; they include apnoea, respiratory distress syndrome, transient tachypnoea of the newborn, pneumothorax, pneumonia and bronchopulmonary dysplasia.
• Patent ductus arteriosus—the ductus arteriosus fails to close in very preterm neonates due to immaturity and as a consequence of the chemical imbalance that results from hypoxia and ventilation–perfusion mismatching. Closure of the ductus is usually achieved by a course of indomethacin, with surgical ligation sometimes being necessary.
• Metabolic disturbances—these include hypoglycaemia, hypocalcaemia, hypomagnesaemia, hyponatraemia and hypokalaemia.
• Susceptibility to infection—this is increased due to low maternal immunoglobulin G (IgG) levels, a less efficient skin barrier, fewer immune cells, and being subjected to more invasive procedures and multiple caregivers.
• Thermal instability—preterm neonates have little or no brown fat stores to maintain their core temperature. In addition, preterm neonates have a larger surface area relative to their size, with thin skin, which facilitates rapid heat loss. Those born at less than 30 weeks gestation also have porous skin, allowing evaporation of fluids. The aim of care,
Box 41.1 Drugs used in the acute resuscitation of the preterm neonate
(Source: Australian Resuscitation Council 2006; Fowlie & McGuire 2005)
• Jaundice—physiological jaundice in preterm neonates is exacerbated due to an immature liver, resulting in delayed conjugation of bilirubin. This will be discussed in more detail below.
• Neurological problems—these include intracranial haemorrhage, the risk factors for which include poor skull ossification, fragile blood vessels and episodes of hypotension, hypertension or hypoxia.
• Gastrointestinal intolerance and necrotising enterocolitis (NEC)—NEC is an inflammatory disease of the bowel usually associated with septicaemia.
• Ophthalmic problems—these include retinopathy of prematurity, with the major risk factor being excessive oxygen exposure or fluctuating quantities; myopia; and strabismus (Henderson & Macdonald 2004; Levene et al 2000).
In addition to the above, preterm neonates also face many another challenges, such as:
• Diminished primitive survival reflexes—these include the suck, swallow and gag reflex.
• Renal immaturity—inability to concentrate urine and excrete an acid load, resulting in late metabolic acidosis.
• Surgical lesions—including the possibility of undescended testes, inguinal and umbilical herniae.
• Haematological problems—this includes haemorrhagic disease of prematurity due to accentuation of the normal neonatal deficiency of vitamin-K-dependent clotting factors; and iron-deficiency anaemia resulting from frequent blood sampling, often requiring a transfusion of packed red blood cells (Henderson & Macdonald 2004; Levene et al 2000).
Small for gestational age
Definition and cause
‘Small for gestational age’ is a term used to describe a neonate whose birthweight, length and/or head circumference is less than the 10th percentile, or greater than 2 standard deviations below the mean, than for the expected gender-specific gestation. The cut-off birthweight for an SGA term neonate is 2500 g. Consideration to variables such as maternal height, weight, parity, ethnicity and geographical location should be considered when using standardised growth charts, as a birthweight below the 10th percentile in one population may not fall below the 10th percentile growth curve in another (Levene et al 2000).
The term IUGR suggests a fetus failing to meet its full fetal growth potential due to factors inhibiting the normal growth process (Groom et al 2007). The most common cause for IUGR is uteroplacental dysfunction resulting in the inadequate delivery of oxygen and nutrients necessary for adequate fetal growth and development of organs and tissues. The terms SGA and IUGR are sometimes used interchangeably; however, these terms are not synonymous. A neonate who is born SGA may not necessarily have suffered from IUGR, and neonates who are born after a short period of IUGR are not necessarily SGA (Lee et al 2003).
Generally speaking, IUGR affects approximately 5% of the general obstetric population and carries an increased risk of perinatal mortality and morbidity. Infants who weigh less than 2500 g at term have a perinatal mortality rate that is 5–30 times greater than that of infants whose birthweights are at the 50th percentile. The mortality rate is 70–100 times higher in infants who weigh less than 1000 g (Peleg et al 1998). IUGR fetuses are at greater risk of stillbirth, birth hypoxia, neonatal complications and impaired neurodevelopment, and possibly type 2 diabetes and hypertension in adult life (RCOG 2002; Sheridan 2005). However, the vast majority of term SGA neonates have no appreciable morbidity or mortality (RCOG 2002).
Approximately 80%–85% of neonates with a birthweight below the 10th percentile for gestational age are constitutionally small but healthy; in the remaining 15%–20%, the cause of IUGR is pathological (Peleg et al 1998; Sheridan 2004). Of the 20% of IUGR neonates, only 10%–15% are ‘true’ IUGR cases, and the remaining 5%–10% are affected by chromosomal/structural anomalies or chronic intrauterine infection (Manning 2004). The causes of ‘true’ IUGR are many, including fetal (multiple pregnancy); maternal (medical factors, socioeconomic and nutritional factors, drugs); and placental factors (recurrent abruption, praevia and immunological disorders) (Sheridan 2005).
The recognition of IUGR involves consideration of risk factors and careful clinical assessment of fetal growth throughout the pregnancy (Manning 2004). If the symphysis fundal height measurement indicates a lag of 4 cm or more, IUGR should be suspected (Bernstein & Gabbe 1996) and further investigations indicated. Fetal growth investigations may include ultrasound, amniotic fluid index (AFI), Doppler studies and biophysical profile (BPP) (Magriples & Copel 2004). Correct postnatal classification of an SGA neonate as being either ‘normal but small’ or ‘IUGR’ requires an accurate assessment of gestational age, correct measurement of birthweight and length at birth, and a precise and comprehensive physical and neurological assessment within 24 hours post-birth. Refer to Chapter 30 for information on physical assessment.
Symmetric and asymmetric SGA neonates
Symmetric SGA neonates experience growth restriction either at the time of conception or during the first trimester of pregnancy, when early fetal cellular hyperplasia is impaired; this produces a proportionate decrease in all fetal organs (Sheridan 2005). The causes for this early onset are often related to fetal factors such as chromosomal or structural anomalies, intrauterine infections, severe maternal vascular disease, severe placental insufficiency, or a constitutionally small neonate (Peleg et al 1998). A symmetric IUGR neonate will usually have weight, head circumference and length all below the 10th percentile with, possibly, some limited brain growth. The neonate may appear active on inspection and demonstrate more-developed neurological responses because of a more advanced age in comparison with size. However, the neonate usually appears malnourished with poor skin turgor, sutures in the skull may be separated widely, and the abdomen may be sunken (Klossner & Hatfield 2006).
Asymmetric growth restriction usually occurs at >32 weeks gestation and commonly results from uteroplacental dysfunction. A fetus experiencing asymmetric growth restriction adapts to a hostile intrauterine environment by redistributing blood flow to the vital organs of the brain, heart and placenta (Peleg et al 1998) in an attempt to preserve brain growth. This gives rise to an SGA neonate with normal head size but a small abdominal circumference caused by decreased liver size; scrawny limbs due to
| Fetal | |
| Maternal | |
| Placental | |
| Environmental | |
| Constitutional | Familial and racial background |
decreased muscle mass; and thinned skin as a result of decreased fat. However, if the insult causing asymmetric growth restriction is sustained long enough or is severe enough, the fetus may lose the ability to compensate and will become symmetrically growth-restricted (Peleg et al 1998). Asymmetrically growth-restricted neonates typically appear thin and pale with loose skin. They have a malnourished, wide-eyed look, with a head that is disproportionately large when compared with the rest of the body. The umbilical cord appears thin and dull-looking, compared with the shiny plump cord of a neonate of average size for gestational age (Klossner & Hatfield 2006).
Labour and birth
Perinatal asphyxia is a risk during labour if IUGR is caused by placental insufficiency, as uterine contractions result in the decrease or cessation of maternal placental perfusion. In addition, perinatal asphyxia may predispose the fetus to meconium aspiration whereby the compromised fetus passes meconium in utero and begins to gasp. (Further information on meconium aspiration can be found later in this chapter in the section on cardiorespiratory conditions.) The fetus should therefore be monitored carefully and continuously during labour. It is also recommended that SGA fetuses are birthed in a maternity unit that has available neonatal expertise and facilities to deal with the associated neonatal morbidities. A healthcare professional skilled in resuscitation should be present at the birth, and where possible a neonatologist should be present if the gestation is extremely preterm or growth restriction is severe (RCOG 2002).
Specific problems
Problems which the SGA neonate may encounter once born include:
• Hypoglycaemia—SGA neonates have decreased tissue glycogen stores, decreased gluconeogenesis and high glucose requirements. Hypoglycaemia may develop any time within the first few days post-birth, particularly when there is a prolonged interval between feeds or nutritional intake is poor. Early enteral and/or intravenous nutrition and glucose monitoring is recommended.
• Temperature instability—SGA neonates have a large surface area and poor subcutaneous fat stores, therefore they should be nursed in a thermoneutral environment.
• Polycythaemia—SGA neonates commonly experience fetal hypoxia which causes increased erythropoietin production. The neonate with polycythaemia at birth appears ruddy and may be tachypnoeic and lethargic.
• Respiratory distress—SGA neonates may experience respiratory distress due to prematurity, meconium aspiration syndrome, or persistent pulmonary hypertension of the newborn.
• Necrotising enterocolitis—SGA neonates, particularly preterms who have had placental insufficiency and abnormal umbilical Doppler studies, are at increased risk.
Outcome
Most infants who are born growth-restricted in utero usually have normal growth rates during infancy and childhood, although studies have demonstrated that at least one-third of SGA IUGR neonates never achieve normal height. A number of studies have also reported an increased risk for low intellectual performance and poor cognitive development among growth-restricted neonates (Bergvall et al 2006; Feldman & Eidelman 2006). Term SGA neonates have no increased risk of severe neurological morbidity compared with the average gestational age (AGA) neonate; however, studies have noted increased hyperactivity, short attention span and learning problems. Preterm SGA neonates, on the other hand, have more minor neurological abnormalities than preterm AGA neonates. The preterm neonates’ neurological development is related to the degree of growth restriction and prematurity. Symmetric SGA neonates are generally smaller and relatively underweight throughout life, whereas asymmetric SGA neonates have an accelerated rate of growth in the first six months followed by normal development.
NUTRITION
Nutrition is vital for adequate neonatal growth, resistance against infection, neurological and cognitive development, and overall long-term health. Nutritional support is paramount to the preterm and sick neonate. Adequate nutrition improves surgical outcome, decreases morbidity and shortens hospital stays (Merenstein & Gardner 2006). The provision of adequate nutrition to a preterm and sick neonate is a challenge to any healthcare professional due to diminished suck–swallow coordination, gastrointestinal immaturity and increased risk of necrotising enterocolitis and acute illnesses; medical interventions, such as umbilical catheters, may also impede the administration of feeds. This section outlines the nutritional requirements, management and risk factors for the preterm and sick neonate.
Feeding methods
Nutritional support can be provided either parenterally or enterally or as a combination of the two. Transitional parenteral nutrition (TPN) is used to supplement enteral nutrition in the form of amino acids to the NICU neonate who is unable to tolerate enteral feeds due to gestation or acute illnesses. Parenteral nutrition is administered intravenously via a percutaneous central venous line. Neonates who have experienced significant asphyxia (hypoxia, metabolic acidosis and hypercapnia) or hypotension may have ischaemic injury to the intestine. Therefore it is not uncommon for these infants to receive parenteral fluids before small feeds are introduced, allowing the potentially compromised gastrointestinal mucosa to recover and avoid complications such as necrotising enterocolitis (Hashim & Guillet 2002). Enteral feeding involves the administration of breast milk or breast-milk substitute into the stomach, stimulating gut hormone secretion and motility and thus assisting the functional adaptation of the gastrointestinal tract (Bombell & McGuire 2008).
Intragastric feeding via an orogastric or nasogastric tube, breast, cup or bottle may be tried, depending on the clinical condition, gestational age and the responses of the neonate. Fetal sucking is seen as early as 13 weeks gestation (Hafstrom & Kjellmer 2000); however, the suck–swallow–breathing coordination is not efficient until nearer term. Preterm neonates of less than 34 weeks gestation are therefore usually fed via an orogastric or nasogastric tube (Hashim & Guillet 2002; Levene et al 2000). Illnesses that increase energy expenditure, such as respiratory or cardiac diseases, or neonates with congenital conditions may also prevent the neonate from suckling, resulting in the provision of intragastric feeds. Intermittent gastric feeds benefit the preterm neonate as they have small gastric capacity and assist the sick neonate in the prevention of respiratory compromise from a full stomach (Henderson & Macdonald 2004).
Clinical point
Neonates are obligatory nose-breathers. Nasogastric tubes can cause partial nasal obstruction, increased airway resistance, and increased work of breathing (Daga et al 1999); therefore an orogastric tube is preferred until respiratory compromise is alleviated.
Intragastric feeding
Intragastric feedings can be introduced minimally (to prime the gut), continuously, or every 1, 2 or 3 hours. Volume and frequency is individualised to the neonate’s gestation and growth curve, clinical condition and gastric emptying time. Gut priming, also known as trophic feeding or minimal enteral nutrition (MEN), involves small amounts of milk feeds (e.g. 1 mL) introduced possibly every 6 hours. This feeding method is commonly used for ELBW neonates, term neonates with mild instability, and or neonates with umbilical catheter placement (Mishra et al 2008). Continuous gastric feeding is generally used for unstable preterm neonates and those with severe respiratory diseases, as well as neonates with delayed gastric emptying time who may not tolerate intermittent gastric feeds (Macagno & Damarini 1994). Administration of continuous feeding is achieved by using an automated pump with the pump rate set at the desired hourly rate. Intermittent 1-, 2- or 3-hour enteral feeds are usually introduced as a progression from minimal or continuous feeds or as the initial commencement of enteral feeds.
Intragastric feeding strategies
Measuring the feeding residual volume before a feed every 4–6 hours (Merenstein & Gardner 2006) can assist in the early identification of gastrointestinal peristaltic paralysis. Although this measurement has not been standardised (Yin 2005
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree