- regulation
the rules and procedures which are issued by government to ensure care is provided in ways that conform with the standards and evidence that are accepted in Australia as defining best practice.
Why does health care need to be regulated, and why can’t we rely on healthcare professionals to regulate themselves? The main reasons why patient safety is regulated are to protect the public from harm occasioned by professional practices that fail to meet national standards, to align health professional behaviours with developments and changes in healthcare systems, and to ensure the health professions constantly improve their performance and that of health service providers (Healy, 2011).
A number of regulatory entities and regulatory mechanisms have been put in place to improve patient safety and enforce national professional standards. Regulatory entities consist of members from the professions, government, the market and society (Healy, 2011; Runciman, Merry & Walton, 2007). Regulatory entities include professional boards; councils and authorities; departments of health; safety and quality commissions; and institutes, insurance funds and consumer groups.
- regulatory entities
organisations formed to regulate health professional behaviour using regulatory mechanisms.
The regulatory mechanisms adopted by these entities to communicate, facilitate and enforce safe, high-quality care range from persuasion through to ‘voluntary strategies’: continuing education, conferences, improvement projects, and the like. Regulatory mechanisms also include ‘command and control’ strategies: criminal and civil penalties for lack of adherence to standards and best practice, and boards where licences to practise may be revoked (Ayers & Braithwaite, 1992; Healy & Braithwaite, 2006; Runciman, Merry & Walton, 2007). Success of these mechanisms depends on a combination of factors. Depending on the issue at hand, workplace culture and the nature of the risk, persuasion may be appropriate; at other times, coercion may be needed.
- regulatory mechanisms
the strategies used to regulate health professional behaviour.
This chapter provides an overview of the regulation of safety and quality and how this influences how healthcare professionals communicate with each other and with their patients. The chapter discusses the main regulatory entities and presents three case studies. Overall, the case studies highlight the relevance and impact of regulation on patient safety and professional behaviour and communication. The first two (in practice example 21.1) provide examples of specific regulatory mechanisms that were employed to regulate health professionals’ clinical behaviour. They highlight the impact of pre-existing organisational culture and human behaviour on the success (or not) of regulatory mechanisms. The third example (in practice example 21.2) focuses more specifically on how poor health professional communication is regulated, and makes reference to the potential disciplinary consequences of poor communication for health professionals.
Introduction
According to Healy (2011) the main regulatory entities in the Australian health sector are government, civil society, the market and the professions.
In relation to government, intergovernmental bodies with responsibilities for safety and quality in health care include the Council of Australian Governments (COAG) and the Standing Council on Health, which comprises health ministers of the states, territories and the commonwealth.
The relevant Commonwealth agencies include the Australian Health Practitioner Regulation Agency (AHPRA), the Australian Commission on Safety and Quality in Health Care (ACSQHC), the Independent Hospital Pricing Authority (IHPA) and the National Health Performance Authority (NHPA). The functions and powers of these agencies is set out in the National Health Reform Act 2011.1
AHPRA is the national organisation responsible for implementing the National Registration and Accreditation Scheme for health providers across Australia. AHPRA works in partnership with the relevant national boards, such as the Medical Board of Australia. AHPRA and the national boards work to regulate the health professions, and they do so in the public interest.
ACSQHC was initially established in 2006 by the Australian, state and territory governments to lead and coordinate national improvements in safety and quality. It became an independent, statutory authority on 1 July 2011. ACSQHC is not a regulatory body, but its legislated functions include developing health service accreditation standards, as well as clinical standards.
IHPA determines the National Efficient Price (NEP) and the National Efficient Cost (NEC) for public hospital services. These price signals ensure that public hospitals receive funding based on ‘unit costs’ – the standard costs of a specific treatment. From 1 July 2012, the Australian government has been using the NEP and NEC to determine its funding contribution to local hospital networks (LHNs; also referred to as ‘local health districts’).
NHPA’s responsibility is to report on the performance of public and private hospitals, primary care networks and community care services right across Australia. These reports can be found on the MyHospitals and MyHealthyCommunities websites (see the ‘Web resources’ section at the end of this chapter).
The Commonwealth Department of Health is the government agency with responsibility for health and health care at a national level. State government agencies include the six state and two territory health departments as well as the various jurisdictions’ safety and quality commissions and councils who advise the department of health.
Coroners, healthcare complaints commissioners, the ombudsman and law courts also have a role in communicating and regulating aspects of safety and quality. The coroner may become involved when there is an unexpected death in a health service that falls within the coroner’s jurisdiction. Complaints commissioners and the ombudsman become involved when there is a formal complaint about a health professional or health service provider. Law courts become involved when there are allegations that the law has been breached. These entities are discussed in more detail in Chapter 23.
The medical professions’ commercial or market behaviour is regulated by the Australian Competition and Consumer Commission (ACCC). The ACCC ensures that health professionals’ commercial interests do not operate in conflict with their professional duties to their clients and the public (Healy, 2011). In the past, the health professions self-regulated via their specific professions council, board and/or college. Because health professional self-regulation was seen to fall short, the professions are now regulated via the federal entity AHPRA. The various professions’ councils, boards and/or colleges still conduct professional conduct hearings and advise the AHPRA.
- professional conduct
all-round proficiency and good conduct.
Outside of governmental agencies and entities, many non-government civil society entities, such as the Consumers Health Forum and interest groups like the Medical Error Action Group, seek to influence and improve how safety and quality are communicated and regulated from a consumer perspective. Most states and territories have local healthcare consumer advocacy bodies.
Responsive regulation
Each of the various entities mentioned utilises a variety of different regulatory mechanisms. Adopting a distinction proposed by John Braithwaite and colleagues (2005), Runciman, Merry & Walton (2007) describe regulatory mechanisms as ranging from ‘soft’ (persuasive) to ‘hard’ (coercive). ‘Soft’ strategies are aimed at encouraging certain behaviour and are voluntary in nature. ‘Hard’ strategies demand and enforce certain behaviour via command and control.
Braithwaite and colleagues (2005) themselves argue that regulatory strategies should be thought of as hierarchical, as depicted in Figure 21.1. Their hierarchy moves from persuasive mechanisms at the bottom of the pyramid to coercive strategies at its top.
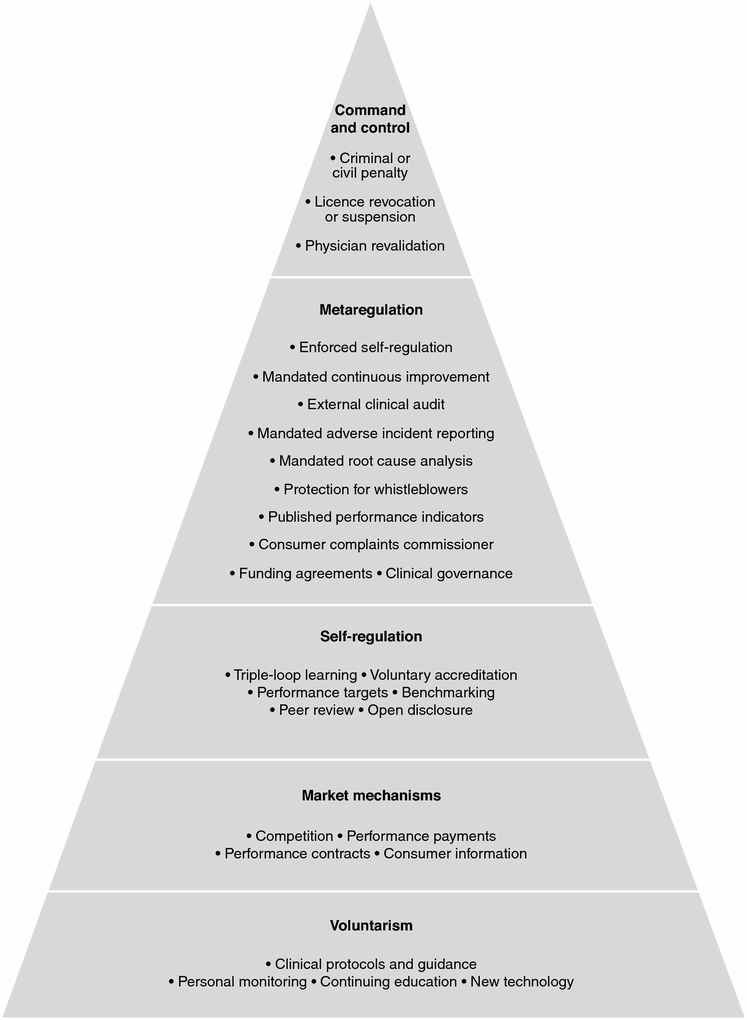
Figure 21.1 The regulatory pyramid and healthcare safety and quality mechanisms (Healy & Braithwaite, 2006)
Figure 21.1 depicts the responsive regulatory pyramid developed by Braithwaite, Healy & Dwan (2005) and Healy & Braithwaite (2006). According to Healy & Braithwaite (2006), regulatory mechanisms should be responsive to the context, conduct, and culture of those being regulated. This is referred to as responsive regulation.
- responsive regulation
the use of mechanisms, strong and weak, such that regulation can be sensitive to the specific contexts and activites that are regulated. This suggests that, rather than issuing only mandatory policies or advisory guidelines, a wide range of regulatory mechanisms whose strength and impact vary is used.
According to Healy and Braithwaite (2006), the key elements of responsive regulation are:
- the regulator first chooses a mechanism from the base of the pyramid using persuasion;
- a single regulatory mechanism is seldom sufficient as the weaknesses of one mechanism must be complemented by the strengths of another;
- there must be the capacity for regulatory escalation to coercion if persuasion fails (Healy & Braithwaite, 2006).
For Braithwaite, Healy & Dwan, responsive regulation means that ‘regulators are more likely to succeed by using [a range of] mechanisms [because] [e]scalating sanctions can be invoked; that is, soft words before hard words, and carrots before sticks’ (2005, p. vii). The authors note that:
The base of the pyramid represents a dialogue-based approach for securing compliance with a just rule or standard where rewards rather than sanctions apply … As we move up the pyramid, more demanding and punitive strategies are invoked. Persistent infractions elicit a formal request to remedy problems, and possibly entail repeat inspections and the public disclosure of failure to meet standards. Closure or removal of license are a last resort, and signal the failure of both the regulator and the regulatee (the person/organisation being regulated) to ensure that the public is well served. (Braithwaite, Healy & Dwan, 2005, p. xi)
Let us now explore how responsive regulation manifests in healthcare practice. As part of our practice example, we explore two UK case studies that mobilised similar regulatory mechanisms but which produced different effects.
This example is based upon research conducted by Ramsay and colleagues (2014) in two UK hospitals. The researchers developed and implemented two ward-level medication safety scorecards in conjunction with staff at two English hospitals. The aim of ‘scorecards’ was to present a visual communication tool to remind staff about safety concerns as outlined on the scorecard. The first scorecard (Scorecard A, Table 21.1) contained medication safety indicators. Non-compliance was coded in red on the scorecard (strikethrough used in table below), and compliance was coded in green (italics). Scores were presented on a weekly basis to staff and each week’s performance was compared with the previous week.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


