12.1 Confidential patient information
For each participant in the study you will need to keep a record of their name, address and other contact information. This can be recorded on a separate form, in a spreadsheet or simply entered into a note book. It is important to store this information separately from the participant’s other data to protect confidentiality. I tend to keep one file with all the patients’ contact information and consent sheets, and a second file for the data collection forms. A unique study number is given to each patient that cross references all the forms.
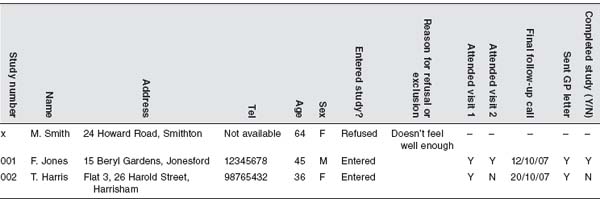
If you are dealing with large numbers of participants it makes sense to keep these records electronically on a spreadsheet. You will be able to keep patients’ names, contact details and demographic details that affect inclusion into the study (like age, sex, or diagnosis) and record the outcome for each person you approach (entered study ineligible, refused, unable to contact). If you are following up participants the spreadsheet will be a valuable tool to record when you contact them, why data couldn’t be collected, and who has completed the study. Such a spreadsheet may look like Table 12.1. If you do this, make sure that you have noted it in your ethics application that you will keep such data and make sure that your spreadsheet is adequately protected to comply with the Data Protection Act 1998.
12.2 Screening form
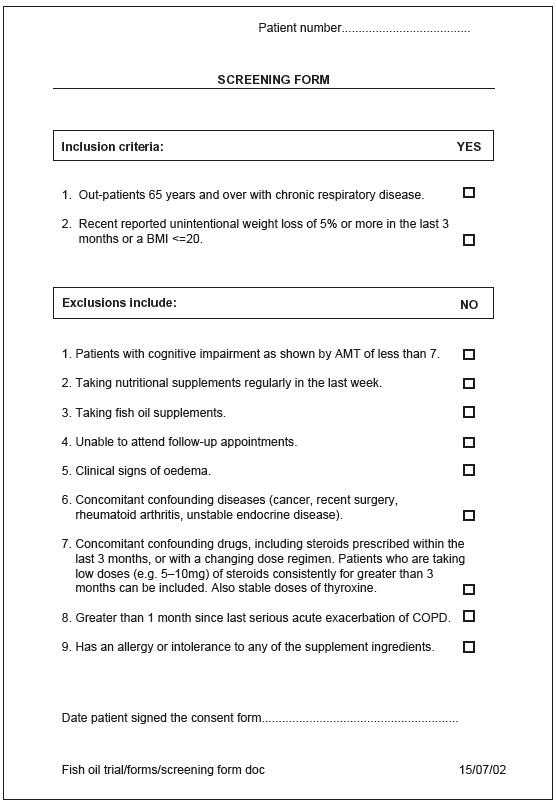
For many studies it is useful to have a screening form, particularly if you have a large number of inclusion and exclusion criteria. The form is simply a list of these criteria so that each can be checked for the patient’s eligibility. You will usually try to complete it before taking consent. However, some screening tests may themselves need consent and so you must delay screening until after consent has been obtained. For example, if you need to assess someone’s muscle strength or blood cholesterol level as part of the screening process you will need to obtain consent first. On the other hand if your criteria relate only to age, where someone lives or whether they have used a particular service you can screen the patient before taking consent.
Table 12.1 Example of a spreadsheet layout containing patient information.
You are more likely to have numerous inclusion and exclusion criteria with quantitative experimental studies. Nevertheless, even if you have very few entry criteria it is still good practice to keep documented evidence that all entry criteria have been met. You may wish to include these on your data collection form, rather than having a separate screening form. An example of a screening form is given in Figure 12.1.
12.3 Data collection
Data collection in quantitative and qualitative research is very different and so requires quite different approaches. Qualitative data are collected using field notes as a supplement to any recorded or printed data. Quantitative data require a highly structured form. Both types of research need demographic data which are best collected on a structured pre-designed form.
12.3.1 Quantitative data collection forms
A good data collection form helps ensure the study is carried out according to the protocol. The following tips will help you collect the right data and carry out the study efficiently:
 Think about the order in which you will do your assessments and make sure the data collection form reflects this.
Think about the order in which you will do your assessments and make sure the data collection form reflects this. Include all demographic and outcome data, as well as other influencing factors, as defined in your protocol.
Include all demographic and outcome data, as well as other influencing factors, as defined in your protocol. Include notes to aid your memory so you don’t miss anything out, for example ‘use the dominant arm for the hand grip test’; ‘book the date for the next interview’.
Include notes to aid your memory so you don’t miss anything out, for example ‘use the dominant arm for the hand grip test’; ‘book the date for the next interview’. Give yourself reminders of tasks that need to be done, such as photocopying consent forms, writing to the GP, giving out instructions or equipment for tests.
Give yourself reminders of tasks that need to be done, such as photocopying consent forms, writing to the GP, giving out instructions or equipment for tests. Include other aidesmemoire such as what colour blood vacutainer to use if you are taking blood, standardised protocols like rest the patient for 5 minutes before taking blood pressure, or phone or bleep number of the person to call if you have a particular problem.
Include other aidesmemoire such as what colour blood vacutainer to use if you are taking blood, standardised protocols like rest the patient for 5 minutes before taking blood pressure, or phone or bleep number of the person to call if you have a particular problem. On every page include the patient’s unique study number and date and use page numbers so you can tell if any sheets get lost.
On every page include the patient’s unique study number and date and use page numbers so you can tell if any sheets get lost. Try it out on the first 2–3 patients, and then make changes; design it to be easy to use.
Try it out on the first 2–3 patients, and then make changes; design it to be easy to use. Ask yourself – could someone else pick it up, use it and get everything done correctly?
Ask yourself – could someone else pick it up, use it and get everything done correctly?An example of part of a data collection form is given in Figure 12.2.
Figure 12.1 An example of a screening form.
12.3.2 Field notes
Field notes are the way qualitative researchers tend to collect observational data and can be regarded as equivalent to the quantitative researchers’ highly structured data collection form. Field notes are generally recorded in a notebook with much less structure but a systematic approach will produce more reliable and understandable data. The following tips from A Data Collector’s Field Guide (Mack et al. 2005; reproduced with permission from Family Health International) will assist you in taking good field notes:
 Begin each notebook entry with the date, time, place, and type of data collection event.
Begin each notebook entry with the date, time, place, and type of data collection event. Leave space on the page for expanding your notes, or plan to expand them on a separate page.
Leave space on the page for expanding your notes, or plan to expand them on a separate page. Take notes strategically. It is usually practical to make only brief notes during data collection. Direct quotes can be especially hard to write down accurately. Rather than try to document every detail or quote, write down key words and phrases that will trigger your memory when you expand notes.
Take notes strategically. It is usually practical to make only brief notes during data collection. Direct quotes can be especially hard to write down accurately. Rather than try to document every detail or quote, write down key words and phrases that will trigger your memory when you expand notes. Use shorthand. Because you will expand and type your notes soon after you write them, it does not matter if you are the only person who can understand your shorthand system. Use abbreviations and acronyms to quickly note what is happening and being said.
Use shorthand. Because you will expand and type your notes soon after you write them, it does not matter if you are the only person who can understand your shorthand system. Use abbreviations and acronyms to quickly note what is happening and being said. Cover a range of observations. In addition to documenting events and informal conversations, note people’s body language, moods, or attitudes; the general environment; interactions among participants; ambiance; and other information that could be relevant.
Cover a range of observations. In addition to documenting events and informal conversations, note people’s body language, moods, or attitudes; the general environment; interactions among participants; ambiance; and other information that could be relevant.12.4 Other forms and equipment
There are various other forms you may need depending on the type of study you are carrying out and the complexity of the study. If you are doing an interventional study, such as a randomised controlled trial, you will need somewhere to record adverse events and protocol deviations. If you are using a questionnaire to collect data this will need to be reproduced for use in your study or developed prior to starting the data collection. If you are using interviews or focus groups you will need to acquire recording equipment. It can also be helpful for all researchers to keep a diary or log of the research activities.
Figure 12.2 An example of part of a data collection form.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


