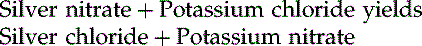6 Chemistry
Scientific Notation, the Metric System, and Temperature Scales
Scientific Notation
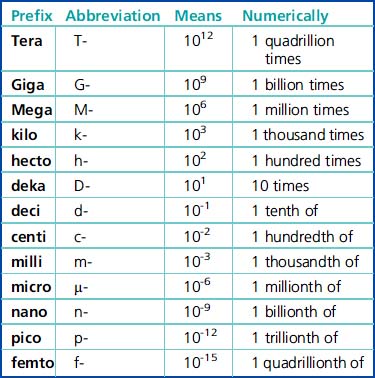
| 109 | 1,000,000,000 |
| 106 | 1,000,000 |
| 103 | 1,000 |
| 102 | 100 |
| 101 | 10 |
| 100 | 1 |
| 10-2 | 0.01 |
| 10-6 | 0.000001 |
| 10-9 | 0.000000001 |
* 1.0 is understood to be the significand with each of the above exponentials.
The Metric System of Measurement
The prefixes are the same and have the same meaning or value, regardless of which basic unit of measurement (grams, liters, or meters) is used. Prefixes are the quantifiers of the measurement units. All of the prefixes are based on multiples of ten. Any one of the prefixes can be combined with one of the basic units of measurement. Some examples are deciliter (dL), kilogram (kg), and millimeter (mm) (Table 6-2).
Temperature Scales
The three most common temperature systems are Fahrenheit, Celsius, and Kelvin.
Kelvin (K) is used only in the scientific community. Kelvin has the following characteristics:
Table 6-3 Important Temperatures in Fahrenheit and Celsius
| Condition | Examples of Fahrenheit (F) and Celsius (C) Temperatures | |
|---|---|---|
| Melting ice | 21° C | 32° F |
| Normal body temperature | 37° C | 98.6° F |
| Boiling water | 100° C | 212° F |
Atomic Structure and the Periodic Table
Atomic Structure
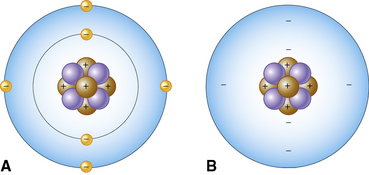
The basic building block of all molecules is the atom. An atom’s physical structure is that of a nucleus and orbits, sometimes called electron clouds. The nucleus is at the center of the atom and is composed of protons and neutrons. At the outermost part of the atom are the orbits of the electrons, which spin around the nucleus at fantastic speeds, forming electron clouds. The speed of the electrons is so great that, in essence, they occupy the space around the nucleus as a cloud rather than as discrete individual locations. The electrons orbit the nucleus at various energy levels called shells or orbits, almost like the layers of an onion. As each orbital is filled to capacity, atoms begin adding electrons to the next orbit. Atoms are most stable when an orbital is full. However, most of the volume of an atom is empty space. See Figure 6-1 for examples of atoms.
The Periodic Table
Matter is defined by its properties. It can also be stated that the properties of matter come from the properties of their composite elements, and the periodic table organizes the elements based on their structure and thus helps predict the properties of each of the elements (Figure 6-2).
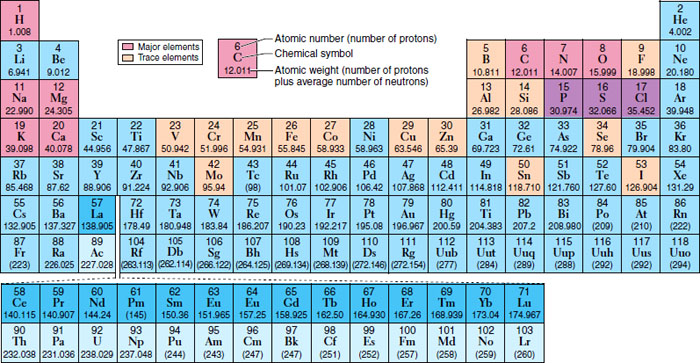
Figure 6-2 Periodic table of elements.
(From Patton KT, Thibodeau GA: Anatomy and physiology, ed 7, St. Louis, 2010, Mosby.)
Chemical Equations
There are also reactions that will create both reactants and products at the same time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree