due to a migraine, not an aneurysm. However, you must evaluate the patient’s headaches in the context of their risk factors and think of radiologic evaluation. JP was a heavy smoker and drinker since 8 years old; in this situation, a CT scan should have been performed on the initial ED encounter to assess for the presence of subarachnoid blood.
TABLE 24-1 COMMON MISDIAGNOSES OF SUBARACHNOID HEMORRHAGE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Small: up to 10 mm
Medium: 10 to 15 mm
Large: 15 to 25 mm
Giant: 25 to 50 mm
Super-giant: larger than 50 mm
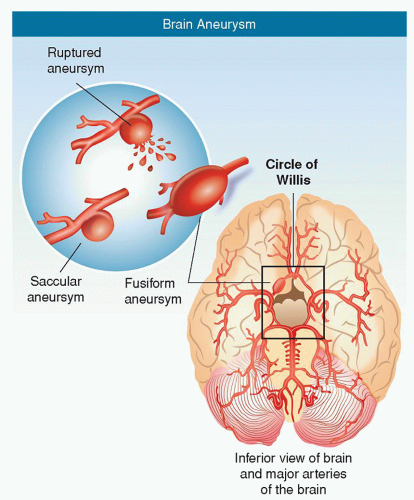
Berry aneurysm: most common type; berry or saccular shaped with a neck or stem (Fig. 24-2)
Fusiform aneurysm: an outpouching of an arterial wall, without a stem (Fig. 24-2)
Traumatic aneurysm: any aneurysm resulting from a traumatic brain injury (accounts for a small number)
Mycotic (infectious) aneurysm: rare; caused by septic emboli from infections, such as bacterial endocarditis; may lead to aneurysmal formation

Figure 24-2 ▪ Aneurysms. (A) Saccular (sac-like), with a well-defined neck. (B) Fusiform (spindle shaped) without a distinct neck. (Copyright 2003 American Stroke Association.)
Charcot-Bouchard aneurysm: microscopic aneurysmal formation associated with hypertension; involves the basal ganglia and brainstem
Dissecting aneurysm: related to atherosclerosis, inflammation, or trauma; an aneurysm in which the intimal layer is pulled away from the medial layer and blood is forced between the layers
85% to 95% in the carotid system, with the following three most common locations.
Anterior communicating artery (Acomm) is the single most common: 30%
Anterior cerebral artery (ACA) are more common in males
Posterior communicating artery (Pcomm): 25% to 30%
Middle cerebral artery (MCA): 20%
5% to 15% in the posterior circulation (vertebrobasilar arteries)
About 10% on the BA: basilar bifurcation, known as basilar tip, most common followed by basilar artery-superior cerebellar artery (SCA) (BA-SCA), basilar artery-vertebral artery (VA) (BA-VA) junction, and anterior inferior cerebellar artery (AICA)
About 5% on the VA and posterior inferior cerebellar artery (PICA) junction is the most common
Fusiform aneurysms are more common in the vertebrobasilar system
20% to 30% of patients who suffer an aneurysm will have multiple aneurysms
a smaller size than those patients with an isolated aneurysm.23 Therefore, treatment considerations are different for patients with an unruptured isolated aneurysm.
several evolving techniques in angiography, magnetic resonance, and ultrasound aimed at the improved imaging of hemodynamic stresses that affect IAs may better define the relationship between the stresses and the vascular remodeling seen in aneurysm wall degeneration and healing.31
 Figure 24-4 ▪ Cross section of the brain with subarachnoid and intracerebral hemorrhage. Asset provided by Anatomical Chart Co. |
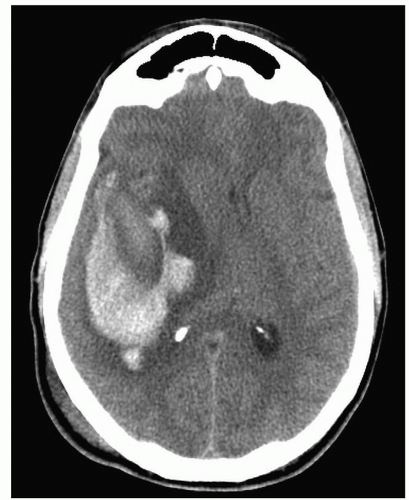
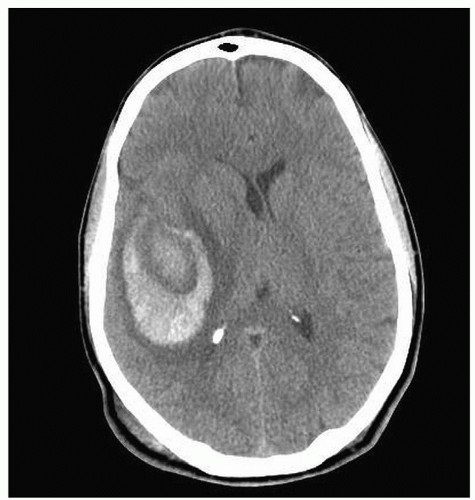 Figure 24-5 ▪ Axial CT scan demonstrating large intraparenchymal hemorrhage within the right temporal lobe. (Courtesy: Christopher S. Ogilvy, MD, Massachusetts General Hospital, Boston, MA.) |
Dilated pupil (loss of light reflex; oculomotor nerve [cranial nerve (CN) III] deficit)
Extraocular movement deficits of the oculomotor (CN III), trochlear (CN IV) or abducens (CN VI) cranial nerves
Possible ptosis (oculomotor nerve [CN III] deficit)
Pain above and behind eye
Localized headache
Nuchal rigidity (neck pain on flexion)
Possible photophobia
Group 1: patients without SAH
Group 2: patients with an earlier SAH from an aneurysm, but with that ruptured aneurysm definitively treated, and having had another unruptured aneurysm
Group 1 patients (no previous SAH) with aneurysms smaller than 10 mm in diameter were 0.05% per year.
Group 1 patients with aneurysms larger than 10 mm in diameter were 20 times higher—approaching 1% per year.
Group 2 patients—smaller aneurysms (i.e., smaller than 10 mm) were 11 times more likely to rupture as aneurysms of the same size in group 1, at about 0.5% per year.
The rupture rate of larger aneurysms (i.e., larger than 10 mm) was similar in group 2 patients compared with those in group 1, at about 1% per year.
information supports a more individualized and sophisticated assessment of the risks of natural history versus the risk of treatment (either surgical or endovascular) on the basis of much more than aneurysm size. With the group 1 patients with aneurysms smaller than 7 mm in size, it is unlikely that the natural history of these lesions can be improved, particularly with older patients and those with anterior circulation aneurysms. Current natural history studies including ISUIA include very few symptomatic patients with small unruptured IAs. An important element in decision making is age of the patient, as age has a major effect on operative morbidity and mortality and very little effect on natural history. It is also important to consider the long-term effects of the efficacy and durability of treatment of both surgical and endovascular procedures and to re-enforce the need for long-term follow-up in these patients.3
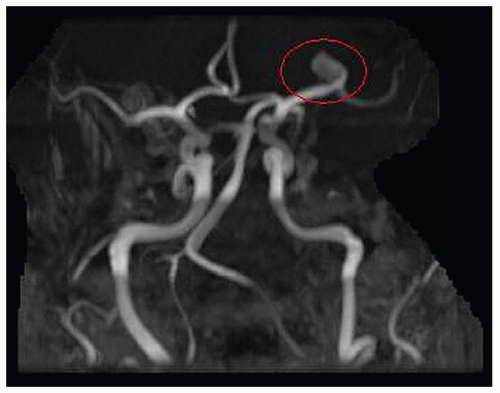 Figure 24-8 ▪ Initial MRA demonstrating only 9-mm L MCA aneurysm without anatomic detail. (Courtesy: Christopher S. Ogilvy, MD, Massachusetts General Hospital, Boston, MA.) |
Cranial nerve deficits (especially CNs III, IV, and VI); non-pupil-sparing CN III palsy produced by expanding Pcomm aneurysm.
Meningeal irritation including nausea, vomiting, stiff neck, pain in the neck and back, and possible blurred vision or photophobia. Signs of meningeal irritation usually appear 4 to 8 hours after the SAH. Mild temperature elevation may accompany SAH.
Stroke syndromes related to the vascular territory involved and intracerebral hemorrhage (e.g., hemiparesis, hemiplegia, aphasia, cognitive deficits).
Cerebral edema and increased ICP resulting in mass effect including seizures, hypertension, bradycardia, and widening pulse pressure.
Pituitary dysfunction (e.g., diabetes insipidus, hyponatremia) secondary to irritation (from blood) or edema resulting from the proximity of the pituitary gland to common locations of aneurysms.
TABLE 24-2 HUNT-HESS SCALE CLASSIFICATION OF SUBARACHNOID HEMORRHAGES | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
neck pain. Presenting symptoms such as confusion or agitation may lead to a diagnosis of a primary psychiatric disorder. Most patients who present with SAH will have an abrupt onset of severe, unique headache or neck pain. Some will have abnormal findings on neurological examinations, such as subtle meningismus or ocular findings. An understanding of the wide presentation of symptoms along with careful history taking and physical examination is the best strategy for identifying patients who should be evaluated for possible SAH.
History and results of the neurological examination.
CT scan, without contrast media; a good-quality fourth-generation CT will detect SAH in 95% or more of cases if scans are done within 48 hours of SAH. Blood appears as high density (i.e., appears white) within the subarachnoid space.
If the CT findings are negative, LP is used in selective cases. RBC counts usually exceed 1,00,000 per mm if a hemorrhage has occurred.
CTA is now being used in many institutions as the first radiographic tool. It is a more recent development than MRA, and its role is being refined, with a reported sensitivity of 95% and specificity of 83%. Unlike conventional angiography, CTA provides a 3D image of the vasculature and demonstrates the relationship to nearby bony structures. With improved techniques in CT scanning, a scan can be completed in a matter of minutes to provide the data necessary to complete 3D imaging of IAs.
MRI is not sensitive within the first 24 to 48 hours, especially with thin layers of blood; it is useful after 4 to 7 days. MRA is also used.
Cerebral angiography remains the “gold standard” for evaluation of cerebral aneurysms. It demonstrates a source of the aneurysm in about 80% to 85% of cases and radiologic vasospasm (i.e., a narrowing of the cerebral blood vessels as seen on radiographic films).
of patients with a diagnosis of SAH who are being transferred to a tertiary care facility for further evaluation and treatment. The imaging time takes about 30 to 40 seconds and can provide immediate confirmation of IA so that treatment decisions can be made rapidly. One disadvantage of the technique is the load of contrast material that is administered which can affect kidney function.
TABLE 24-3 FISHER GRADING SCALE (AMOUNT OF BLOOD ON CT SCAN IS A PREDICTOR OF VASOSPASM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
The examination begins in the location of interest, either the circle of Willis or the carotid bifurcation, using contiguous unenhanced 5-mm thick axial sections. After the area of interest is determined, nonionic contrast is injected intravenously via an automated power injector into an 18-gauge angiocatheter in the antecubital vein at a rate of 5 mL/sec for a total of 120-mL volume, with a 25-second delay between the injection and the onset of data acquisition.52
Two-dimensional (2D) maximum intensity projection (MIP) views and 3D surface-rendered and volume-rendered reconstructions are reformatted from the raw image data. For example, by using this new technology, the Massachusetts General Hospital (MGH) neurovascular group has been able to diagnose and manage approximately 80% of aneurysm patients using CTA alone. This is true for both ruptured and unruptured aneurysms.52
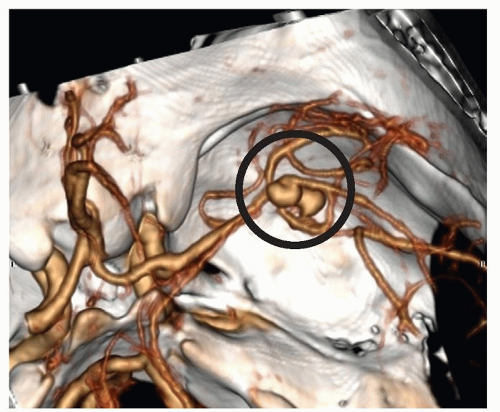
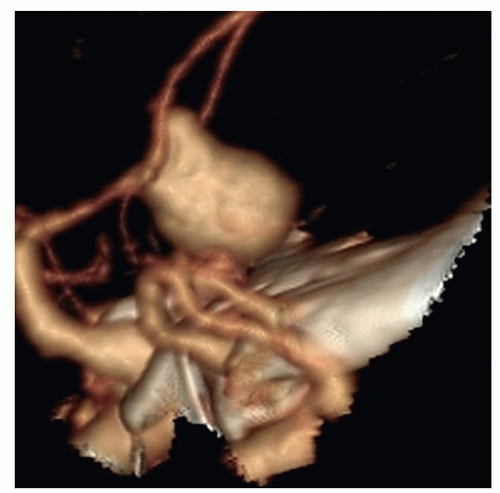 Figure 24-10 ▪ Computed tomography angiogram demonstrating 11-mm anterior communicating artery aneurysm. (Courtesy: Christopher S. Ogilvy, MD, Massachusetts General Hospital, Boston, MA.) |
The particular characteristics of the aneurysm (e.g., shape, size, presence of daughter sac).
Any anomalies of cerebral vasculature (e.g., involving the circle of Willis) that will affect treatment decisions.
The presence of vasospasm, which may influence the urgency to obliterate the aneurysm either surgically or endovascularly, so that triple H (hypervolemic, hemodilution, and hypertensive) therapy or some combination, such as hypervolemic hypertensive management, can be initiated (Dankbaar, Slooter, Rinkel, & van der Schaaf, 2010).
 Figure 24-11 ▪ Conventional cerebral angiogram demonstrating 11-mm anterior communicating artery aneurysm. (Courtesy: Christopher S. Ogilvy, MD, Massachusetts General Hospital, Boston, MA.). |
Confirmation of diagnosis when no aneurysm is found on early angiography (small aneurysms can be missed, particularly in the posterior circulation). Angiogram is usually repeated 10 to 14 days after the initial study.
Identification of reversal of previously observed vasospasm.
Detection and treatment of vasospasm when there is deterioration of neurological function such as change in level of consciousness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree