10 Caring for the patient undergoing cytotoxic therapy
Introduction
Recall the cell cycle in Chapter 2. Re-read this and determine which normal cells in the body are rapidly and continually dividing. Think about what specific impact this might have on the patient undergoing cytotoxic therapy.
There are hundreds of cytotoxic drugs, grouped together according to their biochemical nature (Table 10.1). We also classify the drugs in terms of how they act on the cell. Most cytotoxic drugs disrupt the cell cycle by damaging the DNA or affect mitosis and are classified into groups depending on which part of the cell cycle they affect:
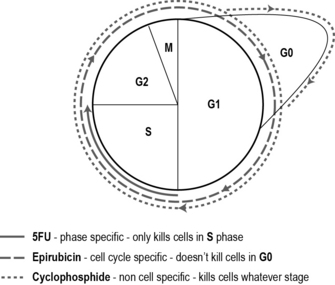
• Cell cycle non-specific drugs kill cells whether in the cycle or resting, i.e. all phases: G0, G1, G2, S and M phases.
• Cell cycle non-phase-specific drugs only kill cells that are active in the cell cycle, i.e. G1, G2, S and M phases, but not G0.
• Cell cycle phase-specific drugs kill cells that are in a particular phase of the cell cycle, i.e. only cells in G1 phase.
Table 10.1 Biochemical classification of cytotoxic drugs
| Groups of cytotoxics | Examples of drugs |
|---|---|
| Antimiotic antibiotics | Doxorubicin Epirubicin |
| Anthracyclines | Mitomycin C |
| Non-anthracyclines | Methotrexate 5-Fluorouracil (5-FU) Vinca alkaloids Capecitabine |
| Antimetabolites | Vincristine |
| Alkylating agents | Cyclophosphamide |
| Taxanes | Taxotere Taxol |
Most cytotoxic drugs are given in combination – usually two or three drugs are used within a regimen. This enhances the effect of the drugs by killing more cells and minimising the range of side effects as well as lessening the risk of cancer cells becoming resistant. For instance, one cell cycle non-specific drug, one cell cycle non-phase-specific and one cell cycle phase-specific drug may be used. Each of the three drugs has different side effects which means the patient is able to tolerate a high dose and a greater tumour kill may be achieved if the drugs all have different modes of action. As an example, the most commonly used chemotherapy regimen for breast cancer after surgery is 5-fluorouracil (5-FU), epirubicin and cyclophosphamide (FEC) (see Fig. 10.1). Sometimes a single drug is used to manage or reduce a patient’s symptoms.
Preparing the patient for cytotoxic treatment
Routes of administration
Watch the peripheral cannulation video at:
http://cancernursing.org/forums/topic.asp?TopicID=74 (accessed November 2011).
Another common method of giving cytotoxic drugs is orally. The number of drugs given this way is increasing due to pharmaceutical developments. It is clearly an attractive method as the patient may receive treatment at home. However, it does result in less contact time from healthcare professionals which may lead to undiagnosed side effects and less psychological support (Irshad & Maisey 2010). There is the issue of concordance (compliance) – patients may not take the tablets as instructed, either forgetting a dose or taking too many. There is also the safety issue of storing medication in the home environment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree