14. Care of the patient requiring cardiac interventions and surgery
Christine Spiers and Kevin Barrett
CHAPTER CONTENTS
Anatomy and physiology of the heart241
Basic pathophysiology of the heart243
Assessment and investigations244
Therapeutic catheterization252
Cardiac treatments256
Open cardiac surgery263
Heart transplantation268
Conclusion271
Introduction
This chapter aims to give an overview of the principles of the care required by patients undergoing a range of invasive investigations and treatments for cardiovascular disease. The more invasive cardiac procedures are reviewed in detail to enhance understanding of what is involved for the patient, so that the nurse is able to tailor information to meet the individual’s needs. Although this chapter describes accepted day-to-day clinical practice, this may differ to local policy and guidelines, and these must be taken into consideration.
Anatomy and physiology of the heart
The heart lies behind the sternum, with two-thirds in the left side of the chest. It is composed of three
layers: the inner layer is the endocardium; the middle muscular layer is the myocardium; and the outer layer is the epicardium. The epicardial surface is surrounded by a protective, inelastic fibrous sac called the pericardium, which contains a small amount of lubricating serous fluid.
At the end of this chapter the reader will be able to:
• describe the structure, function and blood flow through the heart
• identify the structural abnormalities that can affect the heart
• describe how to undertake a nursing assessment of the cardiac patient
• describe the procedure used for cardiac catheterization, its indications, and the nursing management required by patients before and after catheterization
• describe procedures used in the investigation and treatment of patients with cardiac arrhythmias
• identify the specific care required by patients needing temporary and permanent pacing and internal cardiac defibrillators
• discuss the nursing management of patients requiring open cardiac surgery
• identify the specific advice required by patients prior to discharge from hospital following cardiac investigations and therapy.
From the lungs, oxygenated blood enters the left atrium via the pulmonary veins during diastole. Blood flows through the mitral valve into the left ventricle both passively and by contraction of the left atrium. When the ventricle contracts in systole, the mitral valve closes, the aortic valve opens and blood is ejected into the aorta. This is the high-pressure system, as high pressure is necessary to propel the blood through the body. For this reason, the left ventricle has greater muscle mass than the right ventricle. The blood then circulates around the body, becomes deoxygenated and returns once again to the right atrium via the vena cavae.
Contraction of the heart muscle (myocardium) needs to occur in a specific sequence, for these events to occur. This is achieved by the specialized conducting system within the heart. This consists of the sinoatrial (SA) node, which is found at the junction of the superior vena cava with the right atrium, and is the cardiac pacemaker that determines the heart rate. Impulses pass across the atria to the atrioventricular (AV) node at the base of the right atrium and thence to either ventricle via the bundle of His. In the normal heart this is the only route that can be taken by electrical impulses passing from the atria to the ventricles. Impulses are then conducted by the right and left bundle branches to a network of Purkinje fibres which supply the respective ventricles. For practical purposes, events can be regarded as happening simultaneously on both sides of the heart, so that identical volumes of blood flow through both the right and left sides of the heart. Heart rate and strength of contraction are affected by the autonomic nervous system. The heart is richly innervated by sympathetic nerve fibres, which on stimulation increase both the heart rate and speed of electrical transmission via the conducting system. This increases heart rate and strength of contraction. The vagus nerve of the parasympathetic nervous system also innervates the heart and has a broadly opposite effect – namely, slowing the heart rate on stimulation – and can therefore be considered as the heart’s braking system.
Forward blood flow through the heart is maintained by the tricuspid and mitral valves, which prevent the blood flowing backwards from the ventricles to the atria, while the pulmonary and aortic valves prevent blood flowing backwards from the pulmonary artery and aorta into the right and left ventricles, respectively. Although these valves have similar functions, their structures differ. The tricuspid valve, positioned between the right atrium and ventricle, has three cusps, compared with two for the mitral valve, positioned between the left atrium and ventricle. The cusps are thin, strong and fibrous, and when opened are pushed against the ventricular wall to allow blood to flow through them. These valves are attached and stabilized within the heart by specialized parts of the cardiac muscle known as the papillary muscles, which are attached to the tricuspid and mitral valves via cords called chordae tendineae. The two semilunar valves positioned between the right ventricle and pulmonary artery (pulmonary valve) and the left ventricle and aorta (aortic valve) are anchored to the fibrous ring of the cardiac skeleton and do not have papillary muscles or chordae tendineae. Both the pulmonary and aortic valves have three cusps.
The myocardium receives its blood supply from the left and right coronary arteries. These arise from the aorta just above the aortic valve. The left main stem divides into the left anterior descending and circumflex branch. The left anterior descending artery supplies oxygenated blood to the intraventricular septum and the anterior wall of the left ventricle; the circumflex branch supplies the lateral wall of the left ventricle. The right coronary artery supplies the inferior and posterior walls of the left and right ventricles. The myocardium receives its blood supply during the diastolic phase of the cardiac cycle. These vessels also supply the specialized conducting tissue with its oxygenated blood.
Basic pathophysiology of the heart
Any components of the heart can malfunction, either because of congenital abnormality or acquired disease. The heart can also be affected by conditions elsewhere in the body (Table 14.1).
| Myocardium | Pericardium | Coronary arteries | Valves | Conduction system | |
|---|---|---|---|---|---|
| Congenital disease | Atrial/ventricular septal defects Hypertrophic obstructive myocardiopathies | Anatomical abnormalities Homozygous hypercholesterolaemia | Bicuspid atresia | Abnormal pathology Wolff–Parkinson–White syndrome Atrioventricular block | |
| Acquired disease | Myocardial infarction Myocarditis Ventricular septal defect Cardiomyopathies | Pericarditis Tamponade Malignancy | Atherosclerosis Syndrome X | Rheumatic fever Infective Stenosis Regurgitation Papillary muscle rupture Chordae tendineae rupture | Atrioventricular block Sick sinus syndrome AV nodal re-entrant tachycardia |
Coronary heart disease is the most common cause of death in the UK, with 1 in 5 men and 1 in 6 women dying from the disease. Coronary heart disease causes around 101,000 deaths in the UK each year. Other forms of heart disease cause 32,000 deaths each year; thus, in total there were 133,000 deaths from cardiac disease in the UK in 2005 (British Heart Foundation, 2007). There is considerable variation in mortality from coronary heart disease across the UK. Death rates are higher in Scotland and lower in the south-east of England. Death rates are also higher in manual workers than in non-manual workers and are higher in certain ethnic groups. However, death rates from coronary heart disease have fallen considerably in the UK, with an approximate 40% reduction in the last 10 years. Although mortality rates are falling rapidly, the amount of morbidity is not falling. Over 2.6 million people in the UK are living with coronary heart disease, and approximately 1.3 million people have angina, which is the most common presentation of coronary heart disease. In addition, there are just fewer than one million people over the age of 45 years living with heart failure (British Heart Foundation, 2007).
A variety of factors such as smoking, diabetes, sedentary lifestyle, diets high in saturated fats and obesity eventually cause the lumen of the coronary arteries to narrow, causing angina and possible myocardial infarction. The general name for this condition is atherosclerosis. The disease process causing this is due to a variety of complex mechanisms responsible for the accumulation of fatty plaques within the vessel walls and calcification of the vessels; this causes narrowing of the arteries. If the fatty plaques become ulcerated, thrombosis occurs, which may lead to vessel occlusion. The formation of atherosclerosis is essentially an inflammatory process and is caused by a variety of risk factors (Newby and Grubb, 2005). This disease process reduces the blood supply to the myocardium, causing ischaemic chest pain (angina) and, if the artery occludes completely, results in heart muscle death (myocardial infarction). A myocardial infarction can cause additional complications if the valves or conducting system are affected.
There are two main problems which can affect each heart valve.
• If they cannot close properly, they leak, which is called regurgitation.
• When they cannot open properly, they obstruct the flow of blood, and this narrowing is called stenosis.
The valves commonly affected by disease in adults are the aortic and mitral valves. Calcification of the valves due to repetitive mechanical stress can lead to stenosis and stiffening of the valve cusps, which partially obstructs blood flow, and so increases the workload of the heart. More recently, the process of degenerative aortic valve disease has been linked to the risk factors more commonly associated with coronary heart disease, such as hyperlipidaemia, hypertension and smoking (Potts, 2007). If this is not corrected early enough, it may eventually lead to heart failure. Myocardial infarction involving the papillary muscles or causing rupture of the chordae tendineae results in acute mitral regurgitation. This may require urgent surgical repair and/or replacement of the valve.
The conducting system is also affected by a number of conditions which can affect rhythm, resulting in heart rates that can be dangerously fast or slow. Congenital abnormalities such as extra or anomalous conducting pathways (such as Wolff–Parkinson–White syndrome) provide an abnormal connection between the atria and ventricles, which allows the rapid transmission of impulses, causing tachycardia. Ageing, again, can cause fibrosis and calcification of the conducting tissue, which can produce rhythm abnormalities, including various degrees of heart block and slow heart rates. Conduction defects such as heart block may also be caused by coronary artery disease, as a result of occlusion of the artery that supplies blood to the conducting tissue.
Disease processes that affect the myocardium eventually interfere with the pumping action of the heart. This is a frequent cause of heart failure because the heart is no longer able to pump adequate amounts of blood to meet the body’s oxygen demands. This may also lead to myocardial infarction. Heart failure may also result from valve disease, hypertension, pericardial or infective heart disease, or cardiomyopathy. Heart failure is a rapidly increasing problem in the UK, with over 950,000 people diagnosed with definite heart failure (British Heart Foundation, 2007). The condition is very serious, as many patients die within 3 months of initial diagnosis, and the 5-year mortality rate is nearly 60%, which contrasts with a <50% 5-year mortality rate for cancer (McMurray and Stewart, 2000). Except for lung cancer, heart failure has a worse prognosis than all types of cancer, thus illustrating the seriousness of a diagnosis of heart failure for the patient (Stewart et al, 2003).
The cardiomyopathies are categorized as:
• dilated cardiomyopathy
• hypertrophic cardiomyopathy
• arrhythmogenic right ventricular cardiomyopathy or dysplasia
• restrictive cardiomyopathy.
Dilated cardiomyopathy is the most common and has numerous causes, including ischaemic heart disease, hypertension, valvular heart disease and infections; whatever the cause, it eventually results in congestive heart failure, which, when severe, may not respond to anti-failure drug therapy. Hypertrophic cardiomyopathy is thought to be genetically transmitted, resulting in thickening of the intraventricular septum, which eventually causes left ventricular obstruction and may result in sudden cardiac death from arrhythmias (Newby and Grubb, 2005). Arrhythmogenic right ventricular cardiomyopathy or dysplasia is also an inherited disease; it is characterized by fibrofatty replacement of the right ventricle outflow tract. It predisposes individuals to serious ventricular arrhythmias and sudden cardiac death, and may eventually lead to right and then left ventricular failure (Cruickshank, 2004). Restrictive cardiomyopathy is the rarest form and has numerous causes; it results in restricted ventricular filling and reduced ventricular volume. It also causes end-stage heart failure and serious cardiac arrhythmias. Therapy for patients with cardiomyopathy is guided towards the alleviation of symptoms, anti-arrhythmic therapy, treating advancing heart failure, insertion of biventricular pacemakers, internal defibrillators and managing end-of-life heart failure.
Assessment and investigations
Patients with heart disease may require extensive assessment and investigations to diagnose and establish the extent of the disease and its effects on body function. Cardiac assessment should be specific and focused, and may also include risk factor assessment, and exploration of family and social history. The cardinal symptoms of cardiac disease should also be investigated: in particular, questions should be asked about chest pain, breathlessness, palpitations, dizziness, loss of consciousness and ankle oedema.
Cardinal symptoms of cardiac disease
Central chest pain is the most common presenting symptom in coronary heart disease and may also occur in valvular heart disease. It is characterized by radiation to the neck, arms, shoulder, jaw or back and is frequently described as a crushing or vice-like sensation which may come on at rest or after exercise (Fox, 2005). It may also be associated with other symptoms such as nausea, vomiting, aphoresis and extreme anxiety.
Heart disease may cause cardiac arrhythmias and therefore the patient should be asked if they have experienced the following symptoms: palpitations, dizziness or blackouts. These symptoms may be associated with both fast and slow arrhythmias, such as ventricular tachycardia, varying degrees of heart block and atrial fibrillation. These slow or rapid rhythms can result in decreased cardiac output and lowered blood pressure (hypotension), which can, if severe, lead to cardiogenic shock.
Patients with left-sided heart disease often experience breathlessness, either on exertion or at rest. They will often give a history of being unable to lie flat for any length of time, and of wakening from sleep with an episode of acute breathlessness. In addition, the patient should be asked if they have a productive cough and to describe what the sputum looks like. If the patient is expectorating large quantities of frothy, clear/pinkish sputum, this may indicate the presence of heart failure and pulmonary oedema.
The nurse should enquire whether the patient has noticed any ankle swelling, which indicates the presence of oedema and possible right-sided heart failure.
Past history
Previous clinical history is important for both diagnosis and risk stratification of patients. Questions should be asked about past and recent medical history, as many seemingly unrelated conditions can be the cause of the current cardiac complaint. Enquiries about diabetes mellitus should be made, as these patients have a substantially increased risk of coronary heart disease. This risk seems to be higher for women with type 2 diabetes mellitus, and these patients will often have other risk factors for coronary artery disease, such as hypertension and obesity (Jowett and Thompson, 2003). Hypertension, often referred to as the ‘silent killer’, causes heart failure, acute coronary syndrome and, if left untreated, is also linked to renal disease. A history of recent invasive treatment will be of particular importance in a patient with known valvular abnormalities, as it may cause infective endocarditis. The classic ‘invasive treatment’ is usually dental, but genitourinary procedures in particular should be treated as high risk (Newby and Grubb, 2005).
Social and family history
Many cardiac diseases have a familial or genetic component and tactful questioning may elicit this. It is recognized that there are up to 400 sudden arrhythmic deaths in young people each year and recent improvements in technology and clinical skills enable improved prevention, diagnosis and treatment for these at-risk patients In addition, Chapter 8 of the National Service Framework for Coronary Heart Disease (Department of Health, 2005) gives explicit guidance on the investigation of any sudden unexplained death at a young age. Tactful enquiries as to the use of recreational drugs, including cigarettes, alcohol and cocaine, should also be made, as these are all recognized risk factors for coronary heart disease.
Occupational and functional status
Occupation is important, as cardiac disease can also be caused by certain occupations, e.g. publicans may suffer with alcoholic cardiomyopathy, and organic solvents used in the dry cleaning industry are implicated in cardiac arrhythmias and cardiomyopathy. For medicolegal reasons, a diagnosis of cardiac disease can significantly limit, if not completely curtail, a career in the armed forces or police force, and limit the ability of an individual to hold a pilot licence or public service vehicle license (PSV). In addition, the insertion of a cardiac internal defibrillator restricts individuals from driving a Category 1 or 2 vehicle (DVLA, 2008).
Functional capacity should also be assessed, as patients may have deliberately reduced activities such as domestic duties, sporting or other hobbies to limit their symptoms (Douglas et al, 2006). Enquiries regarding the patient’s ability to climb stairs or walk uphill (particularly into cold winds) are particularly revealing, as in some cases coronary artery disease presents predominantly with breathlessness on exertion, associated with climbing inclines.
Risk factors
Cardiac risk factors can be divided into those which are modifiable and those which are non-modifiable. The predominant modifiable and non-modifiable risk factors are listed in Box 14.1.
 Box 14.1
Box 14.1 Cardiac risk factors
Non-modifiable risk factors
Age
Gender
Race
Genetic predisposition
Hyperlipidaemia
Modifiable risk factors
Hypertension
Cigarette smoking
Diabetes
Obesity
Alcohol
Lack of exercise
High blood cholesterol levels
Inquiry into these risk factors and also current drug therapy, including any recreational drugs and over-the-counter medications, should be recorded, as many treatments have cardiac side-effects. This will form a useful basis for any focused health education as part of the cardiac rehabilitation process. It is important to be non-judgemental regarding risk factors, as many patients are fully aware of the role of the risk factors in their current medical problem, and ‘victim blaming’ will only alienate the patient and will not serve as a useful basis for a therapeutic relationship.
Physical assessment
General observation of the patient can reveal useful information such as pallor, cyanosis, shortness of breath at rest and ankle oedema.
The pulse and blood pressure serve as useful tools to assess cardiac output. Measurements of the blood pressure and pulse rate determine whether they are within the normal parameters of:
• pulse rate 60–100 bpm
• systolic blood pressure 100–140 mmHg
• diastolic blood pressure 60–85 mmHg.
A pulse rate below 60 or above 100 bpm may compromise cardiac output, resulting in low blood pressure and insufficient perfusion of body tissues. The quality of the pulse should also be assessed by noting strength and rhythm. Some patients may have an irregularly irregular pulse that could be the result of atrial fibrillation, or an irregular pulse that may indicate ectopic beats.
Further circulatory assessment involves palpating the radial, brachial, femoral, popliteal, dorsalis pedis and posterior tibial pulses to determine their presence and strength. Capillary refill can be used as an indicator of arterial sufficiency, evaluated by applying firm digital pressure to the nail bed to produce blanching; on release of pressure, blood flow should return in less than 3 seconds. This information can be used for comparison of circulatory status after cardiac catheterization.
Many cardiac abnormalities affect respiratory function and vice versa. A thorough respiratory assessment will establish the extent to which the respiratory system is affected. Respiratory assessment should include measurement of the respiratory rate and observation of the depth and rhythm of breathing, respiratory distress, speech pattern and the colour of mucous membranes (Simpson, 2006). Patients having difficulty with breathing frequently use their accessory muscles. This can be seen by observing for retraction of the skin on either side of the neck, just above the clavicles; if breathing difficulty is severe, the skin between the ribs retracts along with abdominal muscle movement. Patients should also be asked whether they experience any breathing difficulty, and which factors cause/ease any breathing problems. Oxygen saturation levels (SaO 2) can be measured with a pulse oximeter, and is a useful indicator of the amount of oxygen bound to the haemoglobin of the blood, which is normally in the range 95–99%. Central cyanosis is not usually detectable until the arterial oxygen tension falls below 8 kPa (kilopascal) and oxygen saturations fall below 90% (Simpson, 2006). The nurse should also enquire whether the patient has a cough, and if sputum is being expectorated, sputum colour, consistency and volume should be noted and recorded.
A detailed history of the patient’s smoking habits should be undertaken, including the following: type and quantity of cigarettes smoked; the period of time of being a smoker; and whether the patient has attempted to stop smoking in the past. This information will help in the formulation of a plan to help the patient give up smoking in the future.
Medical investigations
Some of these investigations are straightforward and, once explained, cause little discomfort for the patient. But the more invasive investigations such as cardiac catheterization can be uncomfortable and frightening and are associated with a number of risks. The following gives an overview of these investigations, followed by a detailed account of the more invasive investigation of cardiac catheterization and the care required by the patient following this procedure.
Chest X-ray
This non-invasive investigation demands little of the patient other than being able to take a deep breath and hold it for a few seconds, long enough for a radiograph to be taken of the chest wall and its contents. This investigation enables assessment of the lungs, heart and great vessels. No specific physical preparation is required, unless the patient is a woman within child-bearing age, in which case information will be required as to when she last menstruated, and whether there is any possibility of her being pregnant, as there is a potential risk that radiation exposure during the radiography could affect the developing fetus.
Blood tests
Blood samples will be obtained for a number of tests: these include estimation of urea and electrolyte levels, which can give an indication of renal function; and full blood count, to assess for anaemia, polycythaemia and infection. Another useful marker of inflammation which is typically raised in infections and autoimmune disease is C-reactive protein (CRP) (Blann, 2007). It has also been suggested that CRP is a strong independent marker of increased cardiovascular risk (Katz and Purcell, 2006). Measurement of potassium is particularly important, since abnormally high or low levels may place the patient at risk of developing cardiac arrhythmias.
Clotting screening is necessary prior to any invasive investigation and treatment if the patient has been taking anticoagulant drugs. If clotting times are prolonged, the patient will be at risk of haemorrhage following any invasive procedure.
Serum cardiac markers are measured if there is suspicion of myocardial infarction. The myocardial-specific isoenzyme of creatinine kinase (CK-MB) and related proteins myoglobin and troponin (troponin T and I) usually remain within the cell; however, if the cell is stressed or damaged, they are released into the circulation. The cardiac troponins are the best markers for definitive diagnosis and are also used to risk stratify patients with chest pain. Normal values for these markers can be obtained through individual hospital laboratories (Blann, 2007).
Electrocardiography
The 12-lead ECG records electrical activity generated by the heart by recording current from leads placed at specific points on the body surface. Different leads look at different areas of the heart, from different directions. The resulting lead patterns give specific information about different anatomical locations of the heart and in particular about conduction disturbances, myocardial perfusion and chamber enlargement. The interpretation of the ECG requires a basic understanding of the physiology underlying the waveforms and an understanding of the ‘view’ ECG leads have of the heart. The interpretation of the ECG is out of the remit of this book, but seeking the help of a knowledgeable senior is always helpful, and many useful ECG textbooks are available for the novice ECG interpreter (Khan, 2004).
Care should be taken to ensure a good-quality tracing, to accurately label the ECG with time and date of the recording and to note the presence and type of chest pain and any other contextual factors.
Ambulatory electrocardiography
Ambulatory ECG recording is used to determine whether a patient’s symptoms are due to an intermittent arrhythmia or to myocardial ischaemia. Also referred to as Holter monitoring, this involves the patient wearing a small tape-recording unit which is attached to three ECG electrodes placed on the patient’s chest wall. The tape recorder continuously records the patient’s ECG for a period of 24–48 hours. The patient is able to activate an event marker that will indicate on the tape when symptoms have been experienced, which can be correlated with the recorded ECG.
Echocardiogram
Transthoracic echocardiography is a non-invasive test which enables the cardiac anatomy and function to be assessed by producing images of the heart from recorded sound waves. The sound waves are recorded from the heart by moving a transducer over the anterior chest wall. Transthoracic echocardiography can identify structural changes of the heart. It can measure the size of the heart chambers, providing information about enlargement or constriction of the ventricle, as well as detecting such abnormalities as the presence of tumours or excess fluid within the pericardial sac (which, if impairing the function of the heart, is known as cardiac tamponade). It is also used to evaluate the functioning of the valves. The test is painless, lasting about 45 minutes, and on completion the patient can resume normal activity.
By comparison, transoesophageal echocardiography is more invasive and therefore associated with some risks. This involves introducing the transducer into the oesophagus, which provides a more direct view of the heart. This test is useful in identifying mitral regurgitation or prolapsed valve, and a dissecting aortic aneurysm. The patient should give informed consent before the procedure, as the procedure involves sedation, fasting and the application of an anaesthetic to the throat to assist insertion of the probe. The patient should therefore be recovered in the normal manner and carefully observed for potential arrhythmias during the recovery period.
Exercise stress (tolerance) test
This is usually performed on a treadmill or bicycle. It employs a continuous 12-lead ECG and blood pressure recordings, and is an invaluable test to reveal cardiac symptoms or ECG changes which may not occur at rest. The test is also used to assess prognosis in patients with known cardiac disease and to evaluate treatments. It is not without risks and should always be performed by trained personnel, with resuscitation equipment nearby (Jowett and Thompson, 2003). A significant proportion of patients cannot perform an exercise test and in these cases an imaging technique such as perfusion scintigraphy may be utilized instead.
Nuclear scans
Nuclear scanning is a rapidly evolving area of medicine and enables the assessment of myocardial perfusion, viability and ventricular function. Single- photon emission computerized tomography (SPECT) involves the intravenous injection of a radioactive substance such as technetium-99 or thallium-201. The substance is taken up by the heart, which is then visualized using a gamma-camera. The radioactive substances enable either myocardial damage (hot-spot detection) or hypoperfusion (cold-spot detection) to be demonstrated. The techniques may be combined with exercise testing for a more accurate method of assessment. Technetium is the increasingly favoured agent used due to its superior image quality. More recently, cardiac magnetic resonance imaging (MRI) has been developed, but, due to limited availability, its use is reserved for evaluation of specific cardiac conditions (Julian et al, 2005).
Cardiac catheterization
This investigative procedure confirms and evaluates the extent of heart disease. The procedure involves gaining access to the heart via major blood vessels. Access to the left side of the heart is achieved by catheterizing either the femoral, radial or brachial artery, while right heart catheterization is performed via the venous route, usually the femoral vein, although the subclavian or jugular veins may also be used. The procedure is performed under local anaesthesia.
Information regarding cardiac function is gained by measuring intracardiac pressures, while X-ray visualization of the heart’s pumping action and blood flow within the coronary arteries, aorta and pulmonary artery is achieved by injecting a radio-opaque dye during X-ray fluoroscopy. This enables visualization of any abnormalities within the chambers of the heart, as well as stenosis or occlusion in the coronary and pulmonary arteries.
Cardiac catheterization is used to confirm and evaluate the progression of the following disorders:
• coronary artery disease
• valve disease
• ventricular dysfunction
• pulmonary and aortic artery disease/disorders
• atrial and ventricular wall defects
• electrical conduction abnormalities.
Catheterization procedure
Both the femoral and radial approaches involve introducing a needle into the artery, followed by threading a long, thin guidewire via the needle into the artery. The needle is removed, leaving the wire, over which an introducer sheath is inserted (Fig. 14.1). During this part of the procedure the patient will experience some discomfort, usually a feeling of pressure over the groin or wrist as the introducer sheath is inserted into the artery, and perhaps mild discomfort as the catheter is advanced along the artery. The patient’s blood pressure and ECG is monitored closely throughout the procedure for any arrhythmias and ischaemic changes.
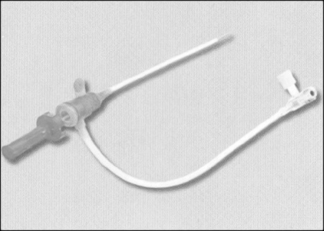 |
| Figure 14.1 • Arterial introducer sheath. |
The introducer sheath allows for repeated access to the artery and heart with different catheters. Within the sheath is a valve that prevents blood loss when the catheter is removed. A catheter can then be threaded up the descending aorta and the tip gently manipulated across the aortic valve into the left ventricle. Radio-opaque dye is injected rapidly into the ventricle (ventriculogram) so that its pumping action can be observed and recorded during X-ray fluoroscopy (Fig. 14.2). This will also identify the presence of abnormalities such as a ventricular aneurysm, valvular regurgitation, or leakage via holes in the septal wall of the heart (atrial or ventricular septal defects).
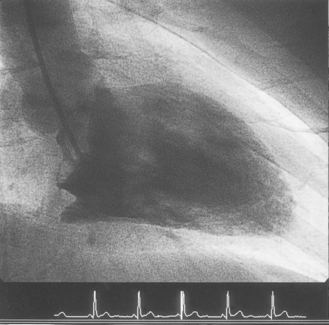 |
| Figure 14.2 • Ventriculogram of the left ventricle during cardiac catheterization. |
During the ventriculogram the patient will experience a ‘hot flushing’ sensation, which may also include a feeling of urinary incontinence. This lasts for a few seconds and is due to the vasodilatory effects of the injected dye. The ventriculogram is followed by manipulating a different catheter into the opening of the coronary artery (Fig. 14.3). Once in position, a small amount of radio-opaque dye is injected into the coronary artery (Fig. 14.4). This will allow the arteries to be visualized by X-ray fluoroscopy and will show any irregularities within the artery, such as areas of stenosis or occlusion. During this part of the procedure, patients do not experience a hot flushing sensation; however, some patients do experience angina, due to partial obstruction of the coronary artery by the catheter and displacement of blood by the dye. The angina usually lasts for a few seconds only, but it is important that the patient informs the staff about the pain so that it can be monitored and treated with a vasodilator such as glyceryl trinitrate (GTN).
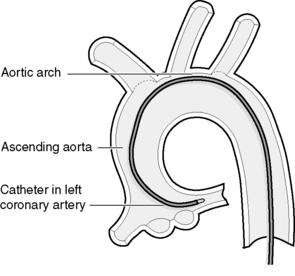 |
| Figure 14.3 • Diagram of a left Judkins-shaped catheter positioned in the ostia of the left coronary artery. |
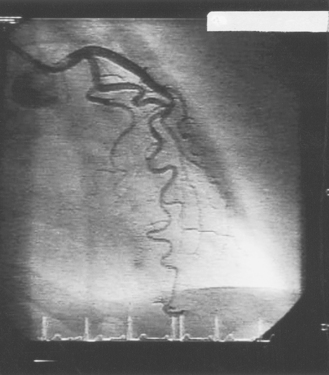 |
| Figure 14.4 • An angiogram of the left coronary artery system. |
The average length of the procedure is between 15 and 30 minutes. On completion, the arterial introducer sheath is removed, and firm pressure is applied 1 cm superior and 1 cm medially to the femoral puncture site to prevent bleeding and promote haemostasis. Various measures are employed to achieve this; one method is to apply digital pressure for 10–20 minutes following arterial sheath removal. Various devices can also be used to promote haemostasis from the femoral artery site, such as pneumatic devices (FemoStop™), suturing and clip devices (Perclose™ and StarClose™), and biodegradable devices which involve implanting a collagen seal into the arterial puncture site (Angio-Seal™) or a seaweed dressing device (Clos-ur™ pads) (Deuling et al, 2008). Although suture and biodegradable devices seal the puncture site rapidly and enable earlier mobilization than does digital compression, the patient continues to require monitoring for complications, as Koreny et al (2004) found an increased risk of haematoma and pseudoaneurysm with these devices. However, on patient comfort, Deuling et al (2008) found that closure devices are more preferable than manual compression.
Haemostasis from the radial artery site can be achieved with digital pressure, or with various devices such as a pneumatic compression device (TR Band™). Radial artery procedures are associated with reduced vascular access complications, earlier patient mobilization, and, as a consequence, shorter hospital admission stay (Archbold et al., 2004 and Steffenino et al., 2006).
Catheterization via the brachial artery differs in that a small skin incision is made over the artery in the antecubital fossa to expose the artery. On completion of the procedure, the arteriotomy is sutured, followed by suturing of the skin incision. Otherwise, the procedure is the same as that described for the femoral approach. Once haemostasis is achieved, the patient is transferred to the ward for further assessment and care.
Electrophysiology studies
Electrophysiology studies are used for patients with symptomatic arrhythmias to assess the conduction system of the heart – namely, the sinoatrial node, atrioventricular node and the Purkinje system. These studies are performed using right heart catheterization procedures similar to those described in the section on cardiac pacing. They are used to identify (and treat) the mechanism of an arrhythmia and the location of the anatomical abnormality (Kaye, 2003). Conditions investigated in this way include supraventricular tachycardias such as those caused by Wolff–Parkinson–White syndrome, atrial flutter and fibrillation, and ventricular tachycardias or bradyarrhythmias. This procedure is used to obtain information about the electrical activity from within the heart, by recording and mapping intracardiac signals during normal sinus rhythm and during the induction of the patient’s arrhythmia (Kaye, 2003). The electrical signals of the cardiac conducting system are recorded by electrodes, which can also pace the heart.
These studies can be lengthy, taking up to 4 hours to complete. The patient is usually conscious but may be lightly sedated during the study. Patients frequently find the procedure uncomfortable because of having to lie still for lengthy periods of time. In addition, patients frequently experience symptoms of their induced arrhythmia, which may be frightening and associated with symptoms such as dizziness, chest pain and palpitations.
Pre- and post-procedural care is similar to that for catheterization, except that patients may need to discontinue antiarrhythmic therapy some time before the study. Cessation of drug therapy leaves the patient without prophylactic protection against arrhythmias. This can cause some patients much anxiety during the period leading up to the study, as it may take many weeks before the body is cleared of some of the drugs.
Pre-procedure assessment and preparation of the patient prior to cardiac catheterization
To enable the patient to give informed consent, the procedure should be explained and consent obtained by the medical staff. Assessment of the patient’s level of anxiety can be used as a gauge to establish the depth of information required about the procedure. Recording baseline observations provides a basis for post-procedure comparison. Assessment includes measuring blood pressure, pulse, ECG, temperature and respiratory rate. The rate and volume of pedal pulses for the femoral approach and/or radial and ulnar pulses for the brachial approach should be assessed. These pulses can sometimes be difficult to find after catheterization, so a small pen mark can indicate their exact position. The colour and temperature of all limbs should be documented, noting the pulse strength and capillary refill times, as these can be useful indicators of arterial sufficiency following the procedure.
Body weight should be recorded, since the dose of many drugs is calculated according to weight: e.g. heparin, which is commonly given during catheterization.
It is necessary to ensure that blood results regarding the patient’s coagulation status and blood chemistry are available prior to catheterization, and that medical staff are informed of any abnormalities. A prolonged prothrombin time may result in bleeding after catheter insertion, whereas abnormal electrolytes may cause cardiac arrhythmias during or after the procedure.
Studies suggest that early education for patients waiting for elective cardiac catheterization may have a positive impact on patients’ quality of life and perceived anxiety (Harkness et al., 2003 and Morkved and Hamilton, 2006).
The patient should be fasted for a minimum of 2–3 hours, as fasting will reduce gastric contents and the risk of inhaling vomit during the procedure, and reduce this risk in the event of complications requiring emergency surgery. Longer fasting periods cause patient discomfort, and dehydration, which can increase the difficulty of gaining venous access.
Management of the patient following cardiac catheterization
The main goals of care after catheterization are early detection of complications and to enhance patient comfort and safety. While cardiac catheterization remains the ‘gold standard’ in assessing coronary anatomy and cardiac function, it does carry a low but definite complication rate (5.8/1000) and mortality rate (0.4/1000) (West et al, 2006).
Whatever the method used for promoting haemostasis, the patient will require regular assessment of vital signs, distal pulses and the skin puncture site for haemorrhage and haematoma formation.
Assessment for impaired circulation of the affected limb includes palpation of the radial and ulnar pulses if the radial or brachial approach was used, and the dorsalis pedis and posterior tibial pulses in the foot if the femoral approach was used. These pulses should be assessed every 15 minutes for the first hour (or according to hospital policy). Presence or absence of the pulse should be assessed, as well as strength and rate. Colour, warmth and sensation of the limb distal to the arterial puncture site should also be assessed regularly according to hospital policy, and Doppler ultrasound may be useful when a pulse is weak or difficult to palpate. A cool, pulseless, pale limb with loss of sensation indicates poor or absent circulation due to interruption of blood flow by arterial occlusion. Capillary refill should be assessed and should be less than 3 seconds.
The introducer site should also be observed regularly for signs of bleeding and/or haematoma formation. If either is evident, a sterile dressing and firm pressure should be applied a few centimetres above the puncture site for approximately 15 minutes or until haemostasis is achieved. Occasionally, large haematomas require surgical evacuation, although most are reabsorbed over a period of time.
Providing the patient has not had any arrhythmias during the procedure, continuous ECG monitoring may not be necessary. If arrhythmias or myocardial ischaemia have been a problem, the patient’s ECG is continuously monitored for further signs of arrhythmias, ischaemic changes and infarction. Vagus nerve stimulation may result in a decrease in pulse rate and myocardial contractility and vasodilatation, causing bradycardia and hypotension. If the heart rate is below 60 beats per minute and associated with hypotension, the immediate goal of care is to increase venous return and therefore blood pressure by placing the patient in a supine position with the lower limbs elevated. If bradycardia persists, the patient may be given atropine, which increases the heart rate and subsequently the blood pressure by inhibiting vagal stimulation (Barbiere, 1994). Intravenous fluids may be given to increase intravascular volume and therefore blood pressure. Atropine may cause angina in some susceptible patients because of the increase in heart rate and subsequent increase in myocardial oxygen demand, but the need for atropine usually outweighs this complication.
Patients may experience discomfort from the needle puncture or incision site, as well as ischaemic pain due to angina. The patient’s level of pain and discomfort should be assessed regularly using a pain scale. Prescribed analgesics should be given as required and their effects evaluated. Pain that is unresolved should be investigated further, as angina unrelieved by vasodilators may indicate myocardial infarction, which requires urgent medical intervention. Pain experienced in the affected limb may indicate haematoma formation, or reduced or occluded blood supply, all of which require immediate attention and appropriate intervention.
Providing the patient is neither nauseated nor drowsy, oral fluids and diet can be recommenced; if the patient’s fluid intake is not restricted, an oral fluid intake of 2.5 L in 24 hours should be encouraged to counteract dehydration due to the diuretic effect of the contrast medium. Increasing fluid intake also increases renal excretion of the contrast medium, which can be toxic to the renal tubules and can cause acute renal failure. Adequate hydration, before and after the procedure, is particularly important for any patient with impaired renal function (ACC, 2001).
Bedrest and immobilization are required for patients after catheterization of the femoral artery, to ensure that haemostasis has been achieved and to minimize haematoma formation. During the period of bedrest the patient should be instructed to keep the affected limb straight and relaxed with minimal movement until haemostasis is established. Duration of bedrest following cardiac catheterization relies on local practice but need be no longer than 2–4 hours, depending on the type of vascular closure device used. Shorter duration of bedrest improves patient comfort and independence. Gentle mobilization can be commenced after the prescribed period of bedrest, providing there are no signs of bleeding or haematoma.
Preparation for discharge
The majority of patients admitted for these investigations have the procedure performed as a day case. Patients should be given appropriate advice before their discharge home, and should be advised to minimize their physical activity (including driving) and to rest for 24 hours following the procedure. They should be advised not to lift heavy objects for 72 hours, to prevent bleeding from the arterial puncture site. If bleeding should occur, the patient or their carer should be advised to apply firm pressure to the puncture site for 15 minutes. If bleeding is not controlled, they should contact their general practitioner or a telephone advice number given by the hospital. Patients should similarly contact their GP or the hospital if the puncture site becomes red, painful or swollen – indicative of infection.
Patients should be advised of the results of their angiography and given further advice about lifestyle modification, management of risk factors and pharmacological therapy. Undergoing cardiac catheterization can be a difficult and anxious time for many patients. Enhancing knowledge and understanding about the procedure and self-care afterwards should reduce anxiety (Morkved and Hamilton, 2006).
Therapeutic catheterization
Patients requiring percutaneous coronary intervention (PCI) and stent implantation or valvuloplasty should be prepared as for cardiac catheterization.
Percutaneous coronary intervention and stent implantation
Percutaneous coronary intervention is a treatment for angina which eliminates or delays the need for coronary artery bypass grafts. The aim of the procedure is to dilate the stenosed or narrowed segment(s) of the coronary artery or, if the artery is completely occluded by thrombus and atheroma, to reopen the artery. Angioplasty and stent implantation widens the diseased vessel lumen and improves blood flow, thus eliminating or reducing the symptoms of angina.
The procedure is similar to cardiac catheterization but differs in that a fine guidewire is introduced into a guiding catheter positioned in the opening of the affected coronary artery. The atraumatic wire is gently advanced into the artery and manipulated across the stenosed or occluded segment. Once the wire is in place, a tiny balloon measuring anything between 1.25 and 4 mm in diameter and between 10 and 30 mm in length (depending on the size of the artery and length of diseased segment) is threaded over the guidewire until it is positioned across the stenosed segment. Once the balloon is in place, it is inflated with a mixture of contrast medium and saline. Inflation of the balloon compresses the atheroma, which may also crack, thus widening the vessel lumen; however, a side-effect of this is local dissection of the arterial medial layer (Roberts, 2001). Widening of the vessel improves blood flow to the myocardium, with cessation or reduction of angina.
Stents are tiny tubes made of a wire mesh which, when expanded by a balloon within them, remain expanded and hold the artery open once the balloon has been deflated and withdrawn (Fig. 14.5). Stents provide structural support for the vessel wall by resisting elastic recoil and hold back any dissected tissue which could otherwise cause the artery to occlude immediately. Although stents have dramatically improved the angiographic results and halved the need for reintervention (Sigwart et al, 2001), they are associated with tissue hyperplasia, leading to in-stent restenosis. This becomes clinically apparent 3–6 months after the procedure, with approximately 10–20% of patients with simple lesions requiring repeat percutaneous coronary intervention (Newby and Grubb, 2005). In an attempt to overcome in-stent restenosis, stents that are coated with a drug that inhibits tissue proliferation into the stent lumen have been developed. These are referred to as drug-eluting stents and are recommended for use dependent upon the anatomy of the target lesion. A recent National Institute for Health and Clinical Excellence (NICE) appraisal committee indicated drug-eluding stents for use in patients in whom the lesion is longer than 15 mm or if the target artery has less than a 3-mm calibre (Curzen, 2008).
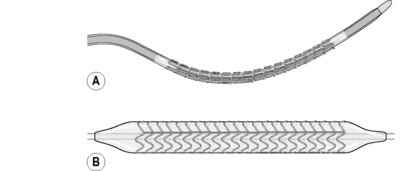 |
| Figure 14.5 • (A) A stent crimped onto a coronary angioplasty balloon. (B) A stent expanded by the balloon. |
The stent comes pre-mounted on a balloon catheter and is introduced into the coronary artery (Fig. 14.6A). When the balloon is inflated, the stent expands and is embedded into the artery wall (Fig.14.6B); the balloon is deflated and removed, leaving the stent behind (Fig. 14.6C). The stent remains in the artery for life to provide structural support, keeping the arterial lumen open. The patient remains conscious but sedated throughout the procedure, which can take as little as 20 minutes (although a complex procedure can take a few hours). During balloon inflation, patients often experience moderate to severe angina caused by total occlusion of blood flow. Pain lasts while the balloon is inflated (Fig. 14.7), usually 15–60 seconds, and subsides on deflation. This is managed with vasodilating drugs such as glyceryl trinitrate. However, if the pain is severe, an opioid such as diamorphine may be given intravenously.
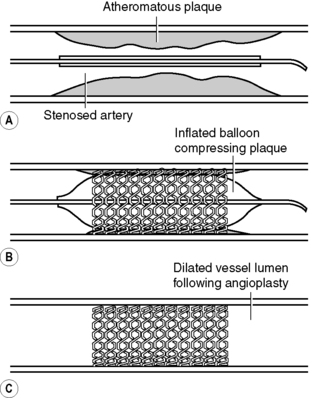 |
| Figure 14.6 • Percutaneous transluminal coronary angioplasty and insertion of an intracoronary stent. (A) Diagrammatic representation of a stenosed artery with a stent mounted on a balloon catheter within the artery lumen. (B) Angioplasty balloon inflated, expanding the stent within the artery wall. (C) Balloon removed, leaving behind the stent to maintain an open vessel lumen to improve blood flow.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
