Chapter 21 Cardiovascular complications of HIV infection
Introduction
The landscape of cardiovascular disease in HIV-infected patients has changed significantly since the introduction of potent antiretroviral therapy (ART). In the early years of the epidemic, the principal cardiovascular manifestations of HIV were dilated cardiomyopathy, pericardial disease, pulmonary hypertension, and neoplastic involvement of the heart. ART has been revolutionary in the care of HIV, significantly reducing the incidence of opportunistic infections and, thereby, prolonging life [1]. However, ART, especially one of its components, the protease inhibitors, can be associated with significant metabolic abnormalities including insulin resistance, lipid abnormalities such as decreased high-density lipoproteins (HDL) and increased triglycerides, and fat redistribution with loss of peripheral fat and intra-abdominal fat accumulation [2]. Therefore, it is not surprising that, as the incidence of dilated cardiomyopathy, pericardial disease, and neoplastic involvement of the heart has decreased, the incidence of cardiovascular disease has increased since the introduction of ART. The prolongation of life with ART and the consequently longer exposure to this therapy and HIV itself may all be factors in the increasing incidence and prevalence of cardiovascular disease in HIV-infected patients.
This review will discuss the most common cardiovascular complications of HIV diseases (Box 21.1), including coronary artery disease, cardiomyopathy and congestive heart failure, pericardial disease, pulmonary hypertension, endocarditis, and neoplastic involvement of the heart. For each of these conditions, there will be a focus on trends in the incidence, prevalence, and disease course from the pre-ART to the current era.
Coronary Artery Disease
With the advent of ART and improved survival with HIV infection, there is now increasing epidemiological overlap between patients with HIV infection and those at risk for coronary artery disease (CAD). In addition to traditional risk factors for CAD, HIV infection itself and certain antiretroviral combinations may also contribute to the increased risk of CAD. Many large cohort studies have found a higher prevalence of traditional CAD risk factors among HIV-infected patients, including higher rates of smoking, lower high-density lipoprotein cholesterol (HDL-C), and higher triglyceride levels [3, 4]. However, the increased risk of CAD events persists despite adjustment for these traditional risk factors [5]. This suggests a role for both the HIV infection itself and, perhaps, certain components of ART in cardiovascular risk. HIV accelerates atherosclerosis through direct effects on cholesterol processing and transport, attraction of monocytes to the intimal wall, and activation of monocytes to induce an inflammatory response and endothelial proliferation [6]. In a large study of patients enrolled in the Kaiser Permanente database, the overall CAD event rate among HIV-infected patients was significantly greater than that of HIV-uninfected controls [7]. Interestingly, the age-adjusted CAD and myocardial infarction (MI) hospitalization rates were not significantly different before and after the introduction of protease inhibitors (PI) or before and after the initiation of other antiretroviral agents. Carotid intimal medial thickness (IMT), a marker of subclinical CAD, is increased in HIV-infected patients compared with those without HIV [8]. The fat redistribution and metabolic change in HIV infection (FRAM) investigators found that the statistically significant increase in carotid IMT in HIV-infected patients persisted after adjustment for both demographics and traditional CAD risk factors [9]. In this study, the effect of HIV on carotid IMT was similar to the effects of male sex, current smoking, and diabetes. In a recent study of non-smoking HIV-infected male patients, duration of HIV infection, not ART use, was associated with increased carotid IMT [10]. Moreover, the rate of MI in HIV-infected patients, though still higher than in controls, has declined in recent years [11]. This reduction in risk is likely associated with increasing use of lipid-lowering therapy in these patients, along with the use of newer ART agents.
In addition to HIV itself, ART has been implicated in increasing the risk of CAD. The HIV Outpatient Study (HOPS), a large prospective observational study, found an increase in the incidence of MI after the introduction of PIs in 1996 [12]. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group, another large prospective observational study, found an increasing incidence of MI with increasing exposure to combination ART [13]. In a follow-up study from the same group, there was an increased risk of MI per year of exposure to PIs [14]. Adjustment for serum lipids attenuated, but did not obliterate, this effect. And, finally, there have been conflicting findings on the association between abacavir use and an increased risk of MI [15, 16]. A recent meta-analysis by the US Food and Drug Administration of 26 randomized control trials showed no increase in the risk of MI with abacavir use [17], whereas a large observational study has suggested a possible link [15]. The expert opinions in the field have concluded that the overall increase in risk of CVD events associated with ART is modest, is outweighed by their effectiveness in controlling HIV disease, and should be combated with modulation of traditional cardiovascular risk factors.
Both HIV and ART are associated with dyslipidemia. After seroconversion, there is a decrease in total cholesterol, low-density lipoprotein cholesterol (LDL-C), and HDL-C [18]. This is followed by increases in levels of triglycerides and very low-density lipoprotein cholesterol (VLDL-C) [19]. In fact, increasing plasma HIV RNA levels are associated with decreasing levels of HDL-C and LDL-C and increasing levels of triglycerides and VLDL-C; decreasing CD4 counts are associated with lower levels of HDL-C [20]. After the initiation of ART, lipid levels return to either baseline levels or higher levels, except HDL-C, which remains low [18]. These changes may represent a general restoration to health or may be the result of direct medication effects. Most PIs raise lipid levels [6]. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are also associated with lipid abnormalities but to a lesser extent than PIs. However, the effects on lipids vary with each specific drug within a class. For example, within the class of PIs, lopinavir/ritonavir and tipranavir/ritonavir are associated with more significant increases in lipid levels, particularly triglycerides, while atazanavir/ritonavir and darunavir/ritonavir cause more modest lipid changes [6]. Several mechanisms by which ART could lead to dyslipidemia have been proposed (Box 21.2) [21].
Box 21.2 Possible mechanisms for protease inhibitor-associated dyslipidemia [21]
LDL, low-density lipoprotein.
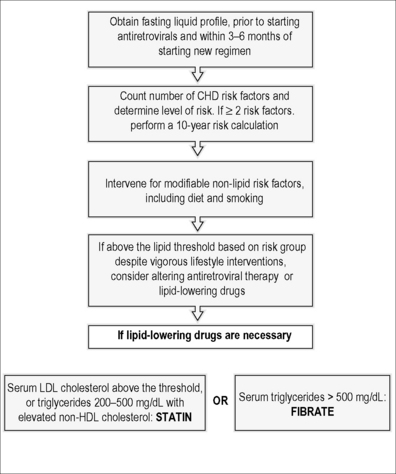
The Infectious Disease Society of America (IDSA) and the Adult AIDS Clinical Trials Group (ACTG) have provided guidelines for the evaluation and management of dyslipidemia in HIV-infected adults receiving ART. These guidelines draw heavily upon the National Cholesterol Education Program Adult Treatment Panel III guidelines. A subsequent implications paper should be considered in conjunction with these guidelines [22]. These topics have been reviewed extensively elsewhere [23] and a summary of these guidelines is provided in Figure 21.1.
Cardiomyopathy and Congestive Heart Failure
Heart muscle disease is one of the most important cardiovascular manifestations of HIV infection. This may present as dilated cardiomyopathy (DCM), myocarditis, or isolated left or right ventricular dysfunction [24]. In the pre-ART era, clinical and pathological studies showed a nearly 30% prevalence of cardiomyopathy in patients with HIV, with an annual incidence of 15.9/1000 cases [25, 26]. DCM carries a poor prognosis in patients with HIV. In a 4-year prospective echocardiographic study survey of 296 patients with HIV infection, DCM was associated with a CD4 count of < 100 cells/mm3 and significantly reduced survival time [27]. DCM tends to occur late in the course of HIV infection. The exact prevalence of myocarditis in patients with HIV infection has been difficult to establish, with estimates ranging from 6 to 52% [26, 28]. It is likely that myocarditis and DCM represent a continuum of disease progression.
Since the introduction of ART, there has been a decline in the incidence and prevalence of DCM. In a single-center retrospective study comparing the prevalence of cardiovascular complications of HIV disease before and after ART, the prevalence of DCM decreased from 8.1 to 1.8% [29]. A more recent report from the Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy (SUN Study) showed a persistently high prevalence of subclinical cardiac structural and functional abnormalities in outpatients with HIV on ART [30]. While nearly two-thirds of the patients had LV systolic or diastolic dysfunction, left ventricular hypertrophy, left atrial enlargement, or pulmonary hypertension, less that 2% had moderate or severe LV systolic dysfunction and 11% had moderate or severe diastolic dysfunction. However, DCM continues to be a significant cardiovascular complication of HIV where access to ART is limited. In a recent study of 416 HIV-infected patients in Rwanda, DCM was documented by echocardiography in 71 (17.7%) of them [31]. By both univariate and multivariate analysis, low socioeconomic status, estimated duration of HIV-1 infection, CD4 count, HIV-1 viral load, CDC stage B and C of HIV disease, and low plasma level of selenium were factors significantly associated with the development of cardiomyopathy.
The pathogenesis of DCM continues to be an area of intense study (Box 21.3). HIV may damage cardiac myocytes by a direct cytolytic effect or through an “innocent bystander” reaction [25, 28]. Infection of myocardial cells by HIV-1 has been demonstrated in a patchy distribution [28]. The mechanism by which HIV infection enters cardiac myocytes, which do not possess CD4 receptors, is not clear. It has been hypothesized that other cells in the myocardium—such as dendritic cells—play a role not only as a reservoir but also as antigen-presenting cells in the context of the major histocompatibility complex and as activators of cytokines that contribute to tissue damage [32]. In a more recent study comparing histopathological sections from HIV-infected patients with and without cardiomyopathy, the expression of HIV envelope protein glycoprotein 120 (gp120) and HIV replication were noted to be more prominent in T-cells and macrophages, which also produce higher levels of pro-inflammatory cytokines, than in cardiac myocytes, in which HIV does not replicate [33]. Although HIV has been detected in cardiac tissue, these findings suggest that an indirect mechanism initiated by inflammatory cells and cytokines or perhaps even a secondary viral infection such as cytomegalovirus, group B Coxsackie virus, Epstein–Barr virus, or adenovirus mediates the high incidence of myocarditis with potential progression to DCM among HIV-infected patients rather than an HIV-specific infection of cardiomyocytes [34].
Box 21.3 Common causes of HIV-associated dilated cardiomyopathy [24]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree