10 Cardiovascular Alterations and Management
After reading this chapter, you should be able to:
• explain the pathophysiology of coronary artery disease, clinical manifestations of acute coronary syndromes and management of events
• discuss the collaborative care for a patient with chest pain
• list the diagnostic tests used to assess myocardial ischaemia
• outline the actions and contraindications of thrombolytic drugs
• outline the clinical manifestations of right and left ventricular failure
• discuss the goals of heart failure treatment
• discuss the pathophysiology of the four different types of cardiomyopathy and how it affects cardiac function
• outline the actions of angiotensin converting enzyme inhibitors, beta-blockers, loop diuretics and spironolactone and how they relate to the pathophysiology of heart failure
Coronary Heart Disease
Coronary heart disease (CHD) is the term used to describe the effects of a reduction or complete obstruction of blood flow through the coronary arteries due to narrowing from atherosclerosis and/or thrombus. Although some patients may be asymptomatic, the commonest manifestations of CHD are chest pain due to angina, acute coronary syndrome (ACS, a term used to collectively describe acute myocardial infarction [AMI] and unstable angina) and sudden death. CHD may also cause arrhythmias and heart failure.1
CHD is the leading cause of death, premature death and disability in Australia and New Zealand.2,3 In 2007, more than 22,000 people died of CHD in Australia, more than 5000 in New Zealand in 2004 and 7.2 million worldwide.2–4 Death rates have fallen by about 76% since the 1960s, primarily due to improvements in risk factors and health care for those at risk. However, the burden of CHD remains high, with 1.5% of Australians reporting CHD symptoms.2 Furthermore, CHD is the single leading health problem worldwide because of a rising incidence in developing countries.4
Myocardial Ischaemia
When coronary blood flow is insufficient to meet myocardial tissue demand for oxygen, myocardial ischaemia occurs. Critical restriction to blood flow occurs when the diameter of the lumen of the blood vessel is reduced by more than half. Coronary blood flow is also determined by perfusion pressure, which can be adversely affected by abnormalities in blood flow (valvular disease), vessel wall (coronary spasm) and the blood (anaemia, polycythaemia).5 Myocardial oxygen demand is influenced by heart rate, strength of myocardial contraction and left ventricular wall tension. As the myocardium receives most of its blood supply during diastole, a rise in heart rate will decrease the duration of diastole and therefore coronary perfusion. Sympathetic stimulation increases the force of contraction and therefore oxygen demand. Left ventricular wall tension increases with the changes in preload associated with filling and afterload associated with systemic vascular resistance. During activity, pyrexia and arrhythmias, these effects may compound due to sympathetic stimulation, causing an increased oxygen demand and reduced coronary perfusion.
Angina
A fixed coronary artery lesion, causing limitation of oxygen supply at times of increased demand, results in stable angina. Therefore, symptoms arise during periods of physical and emotional stress and resolve within 2–10 minutes of rest. Symptoms tend to be worse in the morning (coinciding with a peak in blood pressure), after heavy meals and in cold weather. The severity of symptoms has little correlation with the progress of the disease. However, a patient with a typical history of angina has a high probability of CHD and a higher risk of AMI and sudden death in the following year.6
Unstable Angina and Acute Myocardial Infarction
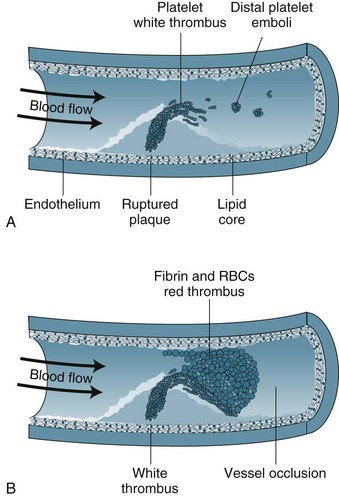
Unstable angina and AMI form a continuum on the basis of reduction in coronary blood flow and subsequent damage to myocardial cells. Unstable angina may indicate transient ischaemia, whereas AMI indicates myocardial tissue death. The term ‘acute coronary syndrome’ (ACS) is now used to represent this continuum.7 ACS results from the rupture or erosion of an atherosclerotic plaque, leading to release of vasoconstrictor substances and potentially triggering coagulation activity (see Figure 10.1). Formation of thrombi results in intermittent and/or prolonged obstruction of the coronary artery. Therefore, ACS typically presents as a recent history of angina (within the past 4–6 weeks); a change in symptoms including increased frequency, more easily provoked or occurring in the absence of physical or emotional stress, more severe or prolonged and/or less responsive to nitrate therapy. ACS is a medical emergency, with up to a third of ACS patients at risk of AMI and death within 3 months.7 There is a high risk of death if the patient experiences more than 20 minutes of pain at rest (pain at rest is associated with changes in ST segment of 1 mm or more on a 12-lead ECG), if there was MI within the previous two weeks, or if pulmonary oedema or mitral regurgitation is present.7
Myocardial Infarction
Myocardial infarction (MI) occurs when blood flow to the myocardium is severely impaired for more than 20 minutes as myocardial cell necrosis begins. Coronary artery thrombus arising from an atherosclerotic plaque is found in the majority of patients dying of AMI.8 Cellular death begins in the subendocardial layer and progresses through the full muscle thickness, so that by 2 hours with total occlusion a full ‘transmural’ infarction will result. However, the full extent of tissue death may occur as a single incident or evolve over several days, depending on the degree of obstruction to blood flow.
The location and impact of the infarction will depend on which coronary artery has been obstructed:
• Left anterior descending (LAD) affects the function of the left ventricle and interventricular septum, including ventricular conduction tissue. Patients with anteroseptal MI are at high risk of heart failure, cardiogenic shock and mortality due to pump deficits.
• Circumflex (CX) affects the left ventricle lateral and posterior walls and the SA node in 50% of people.5 The impact on pump efficiency of lateral and posterior wall necrosis is not as severe as anteroseptal infarcts, although patients are at more risk of arrhythmias.
• Right coronary artery (RCA) affects the inferior wall of the left ventricle and the right ventricle, as well as the AV node in most patients and the SA node in 50% of people. There is potentially severe impact on ventricular function if both the inferior wall and the right ventricle are affected, as well as a high risk of arrhythmias due to SA and AV node involvement.
Patient Assessment and Diagnostic Features
A key feature of assessment of the patient with chest pain is the use of protocols and guidelines to promote rapid assessment so that revascularisation procedures such as thrombolysis and percutaneous coronary intervention (PCI) can be implemented as soon as possible. This means that assessment may begin as early as in the ambulance, with ECG transmission to hospital ED where rapid, early triage models of care are in place.9 Additionally assessment also needs to determine whether there are any contraindications for thrombolysis.
The assessment method used depends on the condition of the patient but should occur within 10 minutes of arrival.7 This initial history will focus on the nature of symptoms such as pain. Pain assessment is complex, and the use of an acronym such as PQRST (see Table 10.1) is useful to incorporate precipitating and palliative factors, qualitative descriptors, location, radiation and length of time. A pain scale is included to help rate the intensity of pain. Asking patients for descriptive words is useful in assessment as many patients will deny pain and instead use words such as pressure, tightness or constriction. It is essential not to ignore other presentations, as patients with atypical symptoms, such as women, often have a delayed diagnosis and treatment and a higher mortality (50%) than with typical symptoms (18%).7 Differentiating this pain from any previous pain is also useful. The brief history should include a short cardiovascular risk profile: (a) previous cardiac history such as angina, MI, revascularisation; and (b) family history, smoking, hypertension, diabetes.
TABLE 10.1 The PQRST criteria for assessing chest pain110
| P | Precipitating | Exercise and activity Stress and anxiety Cold weather |
| Palliating | Stop activity Rest Nitroglycerin | |
| Q | Quality | Heavy, tight, choking, vice-like, constricting |
| R | Region, Radiation | Left side of chest, shoulder, arm and jaw Retrosternal and radiating to the neck |
| S | Severity | Rate pain on scale of 1 (no pain) to 10 (worst pain possible) |
| T | Time | Pain lasts longer than 10 minutes despite nitroglycerin Pain comes and goes but lasts longer than 20 minutes |
Hudak CM, Gallo BM, Morton PG. Critical care nursing, A holistic approach. (7th Ed) Philadelphia: Lippincott 1998.
Practice tip
Because of changes in neuroreceptors, older patients and diabetic patients may not describe the typical anginal pain. Women also may not describe classic angina symptoms and may use different descriptors from men.4 Be alert for prodromal symptoms, such as increased shortness of breath, weakness and fainting.
Electrocardiographic examination
Patients with chest discomfort should be assessed by an appropriately qualified person and have an ECG recorded within 5 minutes of arrival at a healthcare facility to determine the presence and extent of myocardial ischaemia, the risk of adverse events and to provide a baseline for subsequent changes.7 Most importantly, the ECG is essential to determine whether emergency reperfusion is required, and is recommended as the sole test for selecting patients for PCI or thrombolysis. Where ST segment monitoring is available, this should be continuous. Alternatively, if chest discomfort persists, ECGs should be repeated every 15 minutes. Even when chest pain resolves it is important to record a series of 12-lead ECGs during admission to determine changes over time. (The normal ECG is covered in Chapter 9, whereas this section addresses ischaemic changes in the ECG.)
Myocardial ischaemia, injury or infarction cause cellular alterations and affect depolarisation and repolarisation.10 Myocardial ischaemia may be a transient finding on the ECG. Ischaemia results in T wave inversion or ST segment depression in the leads facing the ischaemic area.11 Ischaemic T waves are usually symmetrical, narrower and more pointed. ST segment depression of 1 mm for 0.08 seconds is indicative of ischaemia, especially when forming a sharp angle with an upright T wave.12 These changes are reversible with reduction in demand (e.g. by rest, nitrates).
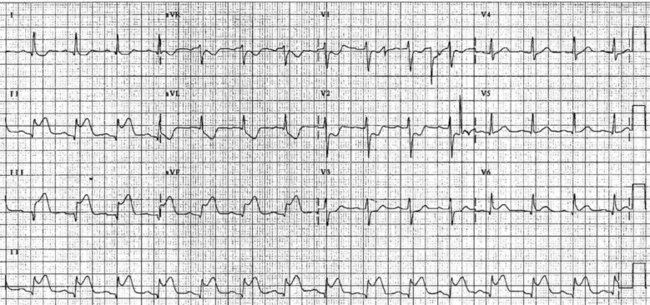
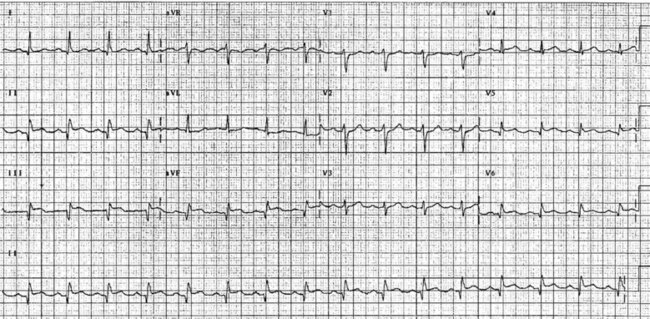
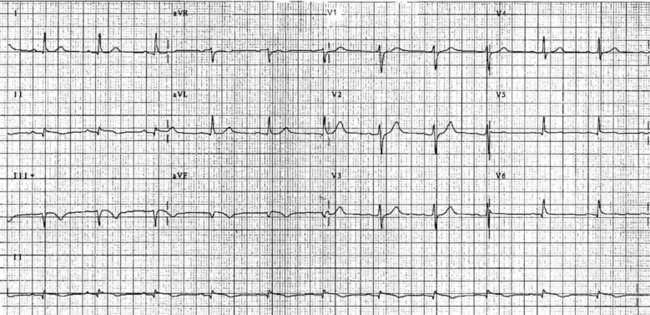
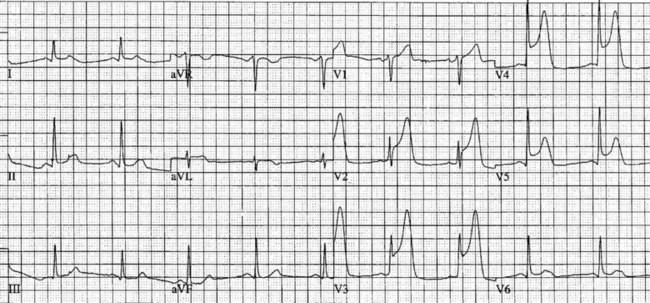
On acute presentation, myocardial injury (infarction) is most commonly associated with ST segment elevation on the ECG, although this is not universal. In addition, a typical pattern of ECG changes over time (evolution of the ST segments, Q wave development and T wave inversion) are often seen (described below), but these changes too are not universal. The distinction between the various acute coronary syndromes, including ST elevation acute coronary syndrome (STEACS), ST elevation myocardial infarction (STEMI) and non-ST elevation myocardial infarction (non-STEMI), is important for ensuring appropriate assessment and protocol-based treatment13 for the various presentations.
• anteroseptal wall of left ventricle, V1–V4;
• anterior wall of the left ventricle, V1-V6, I and aVL;
• lateral wall of left ventricle, I, aVL,V5 and V6;
Additional leads are needed to view the right ventricle and posterior wall. Chest electrodes can be placed on the right chest wall using the same landmarks as the left chest to view the right ventricle (see Chapter 9). Further electrodes, V7–V9, may be placed over the posterior of the left chest to view the posterior wall. Other indicative signs of posterior wall damage are a small r wave in V1 and/or ST depression in V3 and V4 as these may be reciprocal changes. The endocardial surface of the posterior wall faces the praecordial leads of the ECG so the signs of ischaemia and infarction are reversed or reciprocal such as ST depraession or a small r wave. If these signs are present a left-sided ECG, V7–V9, should be done to confirm or rule out a posterior infarction.
Typical ECG evolution pattern
The initial ECG features of myocardial infarction are ST segment elevation with tall T-waves recorded in leads overlying the area of damaged myocardium. These changes gradually change, or evolve, over time, with ST segments returning to baseline (within hours), while Q waves develop (hours to days) and T waves become inverted (days to weeks). The time course for the evolutionary changes is accelerated by reperfusion, e.g. PCI, thrombolysis or surgery. Thus an almost fully-evolved pattern may be seen within hours if successful reperfusion has been undertaken (see Figures 10.2–10.4 for an example). Given the expected time course for evolution, it is possible to approximate how recently infarction has occurred, which is essential in determining management:
• acute (or hyperacute): there is ST elevation but Q waves or T inversion have not yet developed (see Figure 10.5).
• recent: Q waves have developed. ST segment elevation may still be present. Evolution is underway. The infarction is more than 24 hours old.
• old (fully evolved): Q waves and T inversion are present. ST segments are no longer elevated. Infarction occurred anything from a few days to years ago.
Biochemical markers
Intracellular cardiac enzymes enter the blood as ischaemic cells die, and elevated levels are used to confirm myocardial infarction and estimate the extent of cell death. The cardiac troponins T and I (cTnT and cTnI) have been found to be both sensitive and specific measures of cardiac muscle damage.14 Troponin I is rapidly released into the bloodstream, so it is especially useful for the diagnosis and subsequent risk stratification of patients presenting with chest pain in the early stages. Troponin I is also a more appropriate marker to use in postoperative and trauma patients than creatine kinase–MB (CK-MB), as CK-MB levels will be affected by muscle damage. However, CK-MB is less costly and more readily available, and so is still often used, particularly in the presence of a non-diagnostic ECG. C-reactive protein assays may prove to be useful, as baseline and discharge levels are predictive of subsequent cardiac events. However, the laboratory facilities are not readily available.
Coronary angiography and left heart catheterisation
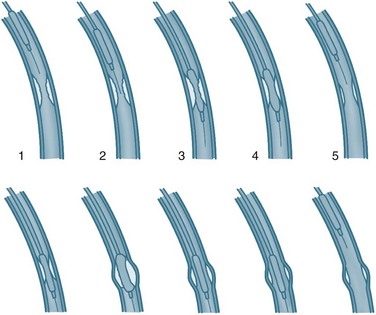
Coronary angiography gives a detailed record of coronary artery anatomy and pathophysiology. Specially designed catheters are advanced with the assistance of a guidewire into the ascending aorta via the femoral or brachial arteries and manoeuvred into the ostium of each coronary artery. Contrast media is then injected and images are taken from several views to provide detailed information on the extent, site and severity of coronary artery lesions and the blood flow into each artery. This flow is graded using the Thrombolysis in Myocardial Infarction (TIMI) studies system (see Table 10.2).15 Typically, a left ventricular angiogram is performed during the same procedure to assess the appearance and function of the left ventricle, mitral and aortic valves. If CHD is present, treatment is determined as appropriate according to the severity (PCI, coronary artery bypass grafting or medical therapy). The nursing care for coronary angiography is similar to PCI, and is covered under that section.
TABLE 10.2 Thrombolysis in Myocardial Infarction (TIMI) flow grades in coronary arteries15
| TIMI 0 | No perfusion and no antegrade flow beyond the occlusion. |
| TIMI 1 | Penetration with minimal perfusion, and contrast does not opacify the entire bed distal to the stenosis during the picture run. |
| TIMI 2 | Partial perfusion and contrast opacifies the entire coronary bed distal to the stenosis, although entry to this area is slower than with unaffected coronary beds. |
| TIMI 3 | Complete perfusion and filling and clearance of contrast is rapid and comparable to other coronary beds. |
Exercise test
Exercise testing with ECG monitoring forms part of the diagnostic screen for patients suspected of stable angina. The Bruce protocol is used most often and considered positive for CHD if there is 1 mm or more of reversible ST segment depression.16 False-positive tests are more common in populations with a lower incidence of CHD, including women.17
Collaborative Management of Angina and Acute Coronary Syndrome
The management of stable angina patients is aimed at: (a) secondary prevention of cardiac events; (b) symptom control with medication; (c) revascularisation; and (d) rehabilitation (see Figure 10.6). (Revascularisation by coronary artery bypass graft is reviewed in Chapter 12; revascularisation by percutaneous coronary angioplasty is reviewed in the next section.)
Treatment of acute coronary syndrome aims at rapid diagnosis and prompt re-establishment of flow through the occluded artery to ensure myocardial perfusion and reduce size of infarction. In addition, treatment aims to:18
• minimise the area of myocardial ischaemia by increasing coronary perfusion and decreasing myocardial workload
• maximise oxygen delivery to tissues
• control pain and sympathetic stimulation
• counter detrimental effects of reperfusion
• preserve ventricular function
The ideal place to manage ACS or MI patients is in the coronary care unit, where continuous, specialised nursing care is available and there is rapid access to treatments.19 Secondary prevention of cardiac events includes the provision of medications, such as antiplatelet therapy and lipid-lowering therapy.
Reperfusion therapy
Thrombolytic therapy
Thrombolytic therapy has been demonstrated to show a significant reduction in mortality in the high-risk group described above.20 The greatest reduction in mortality occurs if the reperfusion occurs within the first ‘golden’ hour of presentation.20 Thrombolysis can be delivered effectively in many settings where other methods of reperfusion are not available.
Streptokinase and tenecteplase are the most commonly prescribed thrombolytic agents. Streptokinase is prepared from beta-haemolytic streptococci and is a potent plasminogen activator.21 Streptokinase is not thrombus-specific, so plasmin is released into the general circulation that may break down any recent clot formed as a result of surgery, injection or healing, leading to a potential increase in haemorrhagic episodes. Streptokinase is bacterial in origin, so it is antigenic. Most individuals have been exposed to beta-haemolytic streptococci so antibodies are often present, which means a higher dose may be required owing to the destruction of some of the enzyme when administered. Occasionally an escalated allergic response will occur and will need urgent treatment. This is more likely if streptokinase has been administered in the previous 6 months. Streptokinase is given intravenously over 60–90 minutes, because it has a short half-life.
The drug tissue-type plasminogen activator (tPA) is available as alteplase, tenecteplase and reteplase. These agents are of human origin, made by recombinant DNA techniques.22 The drug activates only plasminogen present in blood clots, so the risk of haemorrhage is decreased. Unlike streptokinase, tPA can be given repeatedly without risk of anaphylactic reaction. However, tPA costs about 10 times as much as streptokinase, so it is occasionally still reserved for patients who have recently received streptokinase or are at risk of allergic reaction. Often patients with anterior ischaemic changes are treated with tPA (alteplase) based on the GUSTO-1 trial that showed improved outcomes in terms of reduction of ischaemia.23 Alteplase is usually given by infusion, whereas reteplase, which has a longer half-life, can be given in two bolus injections.
• Observations. Assess neurological state including orientation, any IV sites and urinalysis for the presence of bleeding. Along with vital signs, these are attended every 15 minutes for the first hour, half-hourly for an hour, and then hourly according to the patient’s condition, however, patients are advised to report any bleeding postdischarge as well.
• ECG monitoring. This includes 12-lead ECG on return and ongoing ECG monitoring and chest pain assessment to detect reocclusion. Patients need to be requested to inform nursing staff of any chest pain or discomfort.
• IV anticoagulants such as heparin and/or oral antiplatelet drugs, such as clopidogrel or ticlopidine, may be given following thrombolysis to prevent reocclusion in the stent. Assess International Normalised Ratio (INR), prothrombin (PT) and partial thromboplastin time (PTT), as bleeding is more likely to occur if anticoagulants are above the therapeutic range.
Coronary angioplasty
Coronary angioplasty (PTCA) procedures are being used about twice as frequently as coronary artery bypass graft surgery, with 155 PTCA procedures performed for every 100,000 population in Australia in 2008–09.2 PTCA rates have grown dramatically in patients aged over 75 years. In this procedure, a catheter is introduced by the brachial or femoral artery into the coronary arteries and advanced into the area of occlusion or stenosis under the guidance of imagery and specifically designed catheters. A balloon attached to the end of the catheter is then inflated to widen the lumen of the artery by stretching the vessel wall, rupturing the atheromatous plaque and cracking the intima and media of the artery (see Figure 10.7).
PTCA tends to be reserved for patients with single- or double-vessel disease as assessed on coronary artery angiograms. Angioplasty provides better symptom relief than medication alone, but there is no evidence of survival benefits.24 Primary angioplasty results in a higher rate of patency of the affected artery in AMI (>90%), lower rates of CVA and reinfarction and higher short-term survival than thrombolysis.25 PTCA is recommended in all patients presenting with chest pain who meet the indications for reperfusion when: (a) facilities are available and can be achieved within 60 minutes; (b) there are contraindications to fibrinolytic therapy described above; (c) ischaemia would result in large anterior AMI within 4 hours; or (d) haemodynamic instability or cardiogenic shock are present.
A stent is usually inserted to prevent abrupt closure and maintain patency for longer.26 The structure of the stent within the vessel enlarges the lumen and prevents vessel stricture. Restenosis due to intimal hyperplasia is a relatively common complication, occurring 10–12 weeks postimplantation. In response to this problem, drug-eluting stents have been developed. The drug coatings include sirolimus, a macrolide antibiotic that has been demonstrated to effectively decrease hyperplasia and prevent reduction of flow.27 Paclitaxel has also shown promise in a series of studies.28 In addition to dactinomycin, these drugs are undergoing approval processes.
Nursing management of patients post-PTCA includes care of the puncture site to prevent bleeding and detect arterial changes (including clot and aneurysm).29 The process used to create and maintain access for insertion of the catheters can damage the blood vessel(s) and alter perfusion to the limb. The sheath used to aid insertion and maintain access is usually maintained for 1–2 hours post-procedure for emergency access. Care is as follows:
• Observations. Observe access site for haemorrhage and haematoma, assess perfusion to the lower limb, including colour, warmth and pulses. This monitoring needs to be done often in the first few hours, when complications are most likely to occur.
• ECG monitoring. This includes 12-lead ECG on return and ongoing ECG monitoring and chest pain assessment to detect reocclusion. Patients need to be requested to inform nursing staff of any chest pain or discomfort.
• Vital signs. These are recorded every 15 minutes for the first hour, half-hourly for one hour, and then hourly according to the patient’s condition.
• Removal of sheath. This is usually performed by medical or specially trained nursing staff.
• Achievement of haemostasis. Use either application of pressure for at least 5 minutes or vascular sealing.29
• Assess International Normalised Ratio (INR), prothrombin (PT) and partial thromboplastin time (PTT), as bleeding is more likely to occur if anticoagulants are above the therapeutic range. Weight-adjusted heparin (100 units/kg) is usually used during PTCA to prevent thrombus formation, and glycoprotein IIb/IIIa inhibitors such as abciximab may be used to prevent platelet aggregation and thrombus formation for patients at high risk of occlusion.
• Bedrest (2–6 hours) is used to discourage the patient from moving the joint of the insertion site to prevent clot displacement and haematoma formation. Initially the patient should lie relatively flat if femoral artery access has been used, then progress to sitting. The period of rest has been demonstrated to be safely reduced to 1 hour in low-risk patients (normotensive and normal platelet count).29
• Pain relief is used primarily to promote comfort for patients who find bedrest to cause pain and discomfort.
• Urine output. Adequate urine output is essential as radiographic IV contrast is cleared by the kidneys, so it is vital that nurses ensure good hydration and monitor initial urine output.
• Oral antiplatelet drugs, such as clopidogrel or ticlopidine, may be given prior to the procedure to prevent later reocclusion in the stent. Usually patients will be discharged on this medication to continue for up to 3 months while endothelium lines the stent/injured area. Unless contraindicated, all patients will take aspirin for the rest of their lives.30,31
Many patients find the PTCA procedure and confirmation of CHD diagnosis stressful.32 It is an important nursing role to provide patients with preparatory information about the procedure and care required during recovery. As family members provide valuable support and reminders about recovery, these people should be included in any information sessions. The patient and family need to be provided with information about the possibility of restenosis, mobility restrictions at home and the lifestyle changes needed to reduce the risk of worsening CHD.
Nursing management of ACS and MI
Symptom relief should be provided, including analgesia for pain. Analgesia management should be conducted by nurses because of their continued contact and thus more accurate assessment and treatment of pain.18 It is essential to treat pain, not only for the distress it causes patients but also because pain causes stimulation of the sympathetic nervous system (SNS). SNS responses include elevated heart rate and potential for arrhythmias, peripheral vasoconstriction and increased myocardial contractility and, therefore, an overall increase in myocardial oxygen demand. Effective treatments for pain include IV morphine and nitrates. The IV route is preferable, as absorption is predictable and additional punctures in thrombolysed patients are not required. Morphine has the additional benefit of reducing anxiety in a distressing situation and should be initially provided at a dose of 2.5–5 mg at 1 mg/min, followed by 2.5 mg doses as indicated. While there is little randomised controlled trial evidence to support this particular practice, it is generally accepted to be appropriate. A standardised method of pain evaluation and charting should be used to ensure consistent assessment and treatment. An antiemetic such as metoclopramide should be given concurrently to lessen and prevent nausea. Other drugs, such as beta-blockers and nitrates, decrease myocardial workload, contributing to pain reduction.
Nursing care for thrombolysis
Patients receiving thrombolytics require constant observation, regular non-invasive blood pressure measurement for hypotension, and monitoring for allergic reactions to streptokinase. Continuous ECG monitoring for arrhythmias and ST segment changes is essential. Some arrhythmias, particularly idioventricular arrhythmias, are associated with reperfusion and tend to be benign. ST segment monitoring and assessment of pain help evaluate the effectiveness of the thrombolysis. Thrombolysis is considered to have failed if the patient is still in pain and the ST segment has not resolved within 60–90 minutes.18 If thrombolysis fails, patients are at high risk for other interventions, so repeat thrombolysis is often the only treatment option. Salvage or rescue angioplasty may be undertaken if available at the site.
Medications
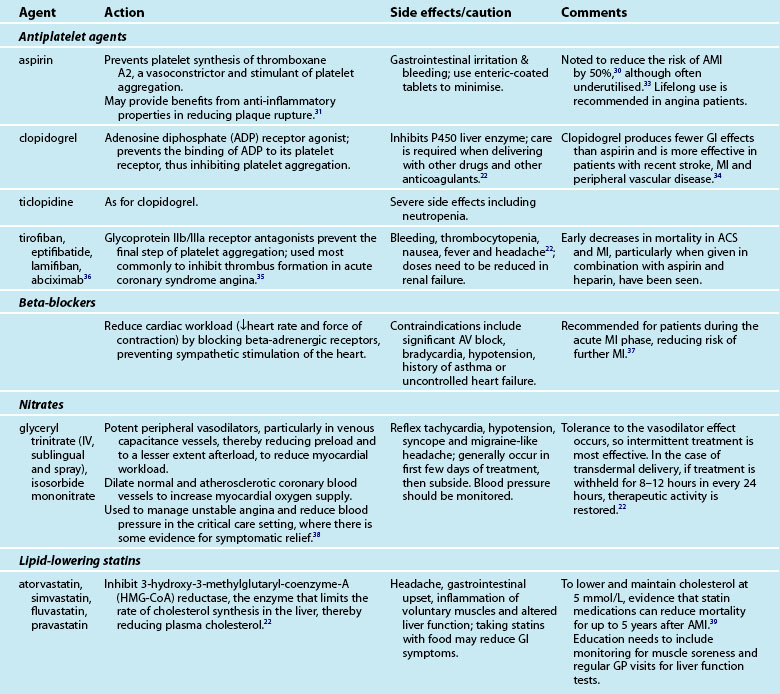
Provision of medications and assessment of the effectiveness of treatment is a major component of the nurse’s role in caring for the cardiac patient. Many of the medications are accompanied by side effects and interactions with other drugs, which the nurse must monitor. An array of medications is used to treat AMI patients, including aspirin, lipid-lowering agents, beta-blockers and organic nitrates (see Table 10.3).
Symptom control
Control of anginal symptoms with medication usually includes sublingual glyceryl trinitrate (GTN) for immediate symptom control and one or more antianginal medications for sustained symptom management.18 Beta-blockers are usually commenced unless contraindicated. Calcium channel blockers may be used in patients who do not have cardiac failure or heart block. (These medications are described in the next section.) The choice of medication may depend on how acceptable the patient finds the reduction in symptoms and the presence of side effects. Patients need to take antianginal agents continuously, regardless of symptoms. Patients should also be encouraged to take sublingual GTN prophylactically.
Angina may also be managed by avoiding situations that trigger angina. Education needs to be directed at awareness of symptoms and management of unstable angina and AMI symptoms, and the need for emergency care. Although these patients are at low risk of further cardiovascular events in the short term, in the medium to long term, risk may accumulate. Patients with angina are encouraged to attend cardiac rehabilitation programs to learn how to deal with symptom management.41
Angiotensin-converting enzyme (ACE) inhibitors have been recommended for all post-AMI patients while in hospital, with review of prescription at 4–6 weeks postdischarge. Patients with left ventricular failure should be maintained on ACE inhibitors. Similarly, diuretics provide the mainstay of the management of left ventricular failure if it is present (see Chapter 19). Diabetic patients have a higher mortality after AMI in both acute and long-term phases. Provision of an insulin-glucose infusion for BSL >11 mmol/L during the acute phase, followed by subcutaneous injections for at least 3 months, has been demonstrated to significantly reduce mortality up to 3 years post-AMI.42
Transfer to a step-down unit or general ward usually occurs when the patient is pain-free and is haemodynamically stable. Stability means that patients are not dependent on IV inotropic or vasoactive support and have no arrhythmias. Discharge home after AMI varies, but usually occurs at day 3 for low-risk patients.18
Independent Practice
Emotional responses and patient and family support
ACS or AMI is usually accompanied by feelings of acute anxiety and fear, as most patients are aware of the significant threat posed to their health.18 For many patients it may also be the first experience of acute illness and associated aspects such as ambulance transport, emergency care and hospitalisation, so they may experience shock and disbelief as well. Fast-track processes require patients and their families to process a large amount of information and make decisions quickly, and this, added to an alien environment, full of unfamiliar technology and personnel, can be quite distressing. However, the environment can also promote a feeling of security for patients and their families. Patients’ perceptions of the CCU environment have been linked to recovery.43
Anxiety is a common response to the stress of an acute cardiac event and leads to important physiological and psychological changes.44 The sympathetic nervous system is stimulated, resulting in increased heart rate, respiration and blood pressure. These responses increase the workload of the heart and therefore myocardial oxygen demand. In an acute cardiac event, these demands occur when perfusion is already poor and may lead to worse outcomes, including ventricular arrhythmias and increased myocardial ischaemia. Therefore, staff working in emergency and coronary care should employ strategies to reduce a patient’s anxiety.
Increasing a patient’s sense of control, calm and confidence in care reduces the patient’s sense of vulnerability, whether it is realistic or not.44 This can be achieved by:
• providing order and predictability in routines, allowing the patient to make choices, providing information and explanations, and including the patient in decision making
• using a calm, confident approach
• communicating with patients and families, while reducing conversation demands as excessive conversation by patients may unnecessarily raise heart rate45
• restricting the number and type of visitors in the acute phase is customary, but many patients feel safer if a family member is present
• provision of comprehensive information to families, with more concise information in understandable language for patients.
Cardiac rehabilitation
Coronary heart disease is a chronic disease process, which often presents with acute events such as ACS or AMI. Like all chronic illnesses, it has implications for patients in terms of lifestyle change, uncertainty of long-term outcomes, functional changes and social and economic alterations. Cardiac rehabilitation aims to address these issues. The World Health Organization describes cardiac rehabilitation as ‘the sum of activities required to influence favourably the underlying cause of the disease, as well as to ensure the patients the best possible physical, mental and social conditions so that they may, by their own efforts, preserve, or resume when lost, as normal a place as possible in the life of the community’.46Systematic, individualised rehabilitation and secondary prevention need to be offered to all AMI patients. Participation in well-structured, multidisciplinary programs has been demonstrated to reduce mortality by up to 30%.47 Additional benefits have been shown for improvements in exercise tolerance, symptoms, serum lipids, psychological wellbeing and cessation of smoking.48–50
Cardiac rehabilitation is structured around four phases, beginning with phase I, during admission.50 The components of phase I include:
• information regarding the disease process, the prognosis, and an optimal approach to recovery, early mobilisation and discharge planning
• assessment of patients’ understanding of their diagnosis and treatment as a foundation for self-management
• discharge planning which incorporates discussions on adaptation to the functional and lifestyle changes needed for secondary prevention – dietary intake of lipids, exercise, smoking cessation, stress management and symptom monitoring, and management of acute symptoms
• early mobilisation as an inpatient to encourage a positive approach to recovery with monitoring of the response to activity in heart rate, shortness of breath and chest pain to determine the rate of progress. (Most hospital units use an activity progress chart for this purpose based on metabolic equivalents [METs]).
The phases that follow, from II to IV, are managed in the outpatient setting and begin with assessment, liaison with multidisciplinary professionals and health education. Phase II occurs in the immediate postdischarge period and includes liaison with community-based carers and services and further assessment. In phase III, tailored, supervised exercise programs are usually conducted and there is a range of psychosocial interventions, such as support sessions and stress management. Finally, in phase IV the focus is on chronic disease management and maintaining risk modification behaviours. All phases require incorporation of the principles of adult learning to maximise learning and behaviour change. These principles include recognition of ‘readiness to learn’.50 Adults are ready to learn most effectively when they are physically and emotionally stable and are aware of the problem or need to learn. Nurses, because of their expertise and continual presence, are best placed to assess and provide education at optimal times.
Complications of Myocardial Infarction
Cardiogenic shock
Cardiogenic shock occurs as a complication of MI in about 5–10% of patients and is the most common cause of death in hospitals.50 It arises from loss of contractile force, and generally occurs when ventricular damage is more than 40% and ejection fraction less than 35%. Cardiogenic shock and the related management are described in more detail in Chapter 12.
Arrhythmias
Arrhythmias often occur in ACS and AMI and are often the cause of death in the prehospital phase. Management of the prehospital phase centres on community education and an effective, rapidly responsive ambulance service, as exemplified in Seattle in the USA.51 Arrhythmias may be generated by poorly perfused tissue and electrolyte alterations, and increased sympathetic tone during infarction, but are more often due to a failing left ventricle. They may also complicate reperfusion after successful revascularisation.52 It is essential to rapidly and effectively treat arrhythmias in the ACS and AMI context. The goal of treatment is to maintain cardiac output while reducing workload. Arrhythmias and management are described in Chapter 11.
Pericarditis
Pericarditis is an inflammation of the visceral and parietal layers of the pericardium that cover the heart. This inflammation occurs in approximately 20% of AMI patients within the following 2–3 days.10 The patient experiences chest pain, which may be confused with ischaemic pain. This confusion with an ischaemic event may be compounded by the additional presence of ST segment elevation on the ECG. However, pericardial pain increases with deep inspiration and a pericardial rub is often present. Electrocardiographically, the elevated ST segments of pericarditis are typically concave upwards (saddle-shaped) and often widespread, contrasting with convex ST segment elevation limited to the distribution of a single coronary artery in infarction.53 Pericarditis normally responds to anti-inflammatory treatment by aspirin, indomethacin and/or corticosteroids. Approximately 1–5% of AMI patients develop pericarditis as a late complication, 2 weeks to a few months post-AMI.18 Usually this late-onset pericarditis is associated with Dressler’s syndrome and may be an autoimmune response to myocardial injury. This is a chronic condition requiring systemic corticosteroid treatment.
Structural defects
Papillary muscle rupture most often occurs 2–7 days after MI. Patients experience a sudden onset of pulmonary oedema secondary to pulmonary hypertension and cardiogenic shock. Additional heart sounds and a systolic murmur will be heard. Urgent surgery is required, as the mortality rate for papillary muscle rupture is 95%.54 Cardiac rupture most often occurs within 5 days of MI and is commonest in older women. The patient experiences continuous chest pain, dyspnoea and hypotension as tamponade develops. Symptoms may worsen rapidly and result in pulseless electrical activity (PEA) unless surgery is undertaken immediately.
Heart Failure
In normal circumstances, the heart is a very effective, efficient pump with reserve mechanisms available to allow output to meet changing demands. These mechanisms include (a) increasing heart rate to increase total cardiac output, (b) dilation to create muscle stretch and more effective contraction, (c) hypertrophy of myocytes over time to generate more force, and (d) increasing stroke volume by increasing venous return and increased contractility. Heart failure is a complex clinical condition that is characterised by an underlying structural abnormality or dysfunction that results in the inability of the ventricle to fill with or eject blood.55 The condition is also known as congestive cardiac failure, a term commonly used in the USA but not in Australia. Chronic heart failure (CHF) describes the long-term inability of the heart to meet metabolic demands.
The burden of disease associated with heart failure is on the rise due to our ageing population, the prevalence of coronary heart disease and hypertension, the decrease in fatality from acute coronary syndrome and improved methods of diagnosis.55 Survival rates and prognosis for heart failure patients are extremely poor. Approximately 50% of patients diagnosed with heart failure will die within five years of diagnosis.56–58 When compared with those patients with cancer, heart failure patients have the poorest five-year survival rate, with the exception of lung cancer.59 In Australia during 2001–2002, 41,874 patients were hospitalised with a primary diagnosis of CHF (0.7% of all hospitalisations).60 Internationally, heart failure is the most common cause of hospitalisation in patients aged over 70 years.55 Approximately 40% of patients admitted to hospital with heart failure will be readmitted or die within one year.61
Over 50% of patients newly diagnosed with heart failure have concurrent ischaemic heart disease, hypertension is present in 65% and idiopathic dilated cardiomyopathy (5–10% of cases).55 The causes of heart failure can be categorised according to (a) myocardial disease, (b) arrhythmias, (c) valve disease, (d) pericardial disease and (e) congenital heart disease.62 Myocardial disease may be caused by myocardial infarction and fibrosis from prolonged ischaemic heart disease which accounts for approximately two-thirds of systolic heart failure causing systolic dysfunction and a reduced ejection fraction.
Arrhythmias, including both brady- and tachyarrhythmias, may cause heart failure due to changes in filling time affecting preload and resultant cardiac output. Myocardial oxygen demand is increased and if the heart is poorly perfused, muscle contraction will be affected. Frequent premature contractions and atrial fibrillation disturb mechanical coordination so that the ventricles may not be adequately filled for efficient contraction. Heart failure patients are also at high risk of sudden cardiac death due to ventricular fibrillation or tachycardia. Valvular disease causing heart failure usually involves valves on the left side of the heart (mitral and/or aortic valves). Aortic stenosis results in an increase in afterload and ventricular hypertrophy develops with reduced diastolic compliance resulting in a reduced ejection fraction. Mitral stenosis is usually due to rheumatic heart disease. Valvular incompetence results in a dilated ventricle to accommodate the regurgitant volume. Stroke volume increases in an attempt to empty its contents and ventricular muscle mass increases. However, over time the ventricle is unable to maintain the increased workload and heart failure develops. Valvular heart disease and treatment is described in more detail in Chapter 12.
• Backward failure: refers to the systemic and pulmonary congestion that occurs as a result of failure of the ventricle to expel its volume.
• Forward failure: is due to an inadequate cardiac output and leads to decrease in vital organ perfusion.
• Acute heart failure: includes the initial hospitalisation for the diagnosis of heart failure and exacerbations of chronic heart failure.
• Chronic heart failure: develops over time as a result of the inability of compensatory mechanisms to maintain an adequate cardiac output to meet metabolic demands.
• Systolic heart failure: refers to the inability of the ventricle to contract adequately during systole resulting in a reduced ejection fraction and an increased end-diastolic volume. This is the most common form of heart failure.
• Diastolic heart failure (or heart failure with preserved systolic function [HFSF]): indicates normal systolic function with a normal ejection fraction but impaired relaxation so there is a resistance to filling with increased filling pressures. Diastolic dysfunction usually occurs in conjunction with systolic dysfunction and is more common in the elderly.
• Low cardiac output syndrome: this occurs in response to hypovolaemia and/or hypertension. Severe vasoconstriction further reduces the cardiac output.
• High cardiac output syndrome is the result of an increase in metabolic demands causing a decrease in SVR leading to an increase in stroke volume and cardiac output. Burns and sepsis are the main causes.
• Left sided heart failure: occurs when there is a reduced left ventricular stroke volume resulting in accumulation of blood in the pulmonary system.
• Right sided heart failure: is the congestion of blood in the systemic system due to the inability of the right ventricle to expel its blood volume.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree