11 Cardiac Rhythm Assessment and Management
After reading this chapter, you should be able to:
• describe the various arrhythmogenic mechanisms implicated in the development and propogation of cardiac arrhythmias
• recognise the features of the various commonly observed arrhythmias and discuss the aetiological factors that predispose to the development of each
• discuss the actual or potential haemodynamic consequences and prognostic implications of each of the commonly observed arrhythmia types
• describe the general and specific assessment and treatment strategies applicable to each of the various arrhythmia types
• discuss the principles and indications for pacemaker therapy
• recognise abnormal pacemaker activity on ECG and discuss the causes and corrective actions for complications during temporary pacing
• describe the principles and benefits of cardiac resynchronisation therapy (CRT), including the factors which limit the effectiveness of the therapy
• discuss the principles and indications for treatment of arrhythmias including ablation therapies, permanent pacing, cardioverter defibrillators, cardioversion and defibrillation.
The Cardiac Conduction System
The normal heartbeat sequence occurs through rhythmic stimulation of the heart via its specialised conduction system. The sinoatrial node, located superiorly in the right atrium, spontaneously generates an activation current that conducts across preferential right and left atrial pathways (producing a P wave on the surface ECG) and then to the atrioventricular node at the lower interatrial septum. After a brief physiological slowing of the current (to allow the ventricles to be optimally ‘pre-loaded’), the impulse travels to the Bundle of His in the upper interventricular septum before spreading down through the ventricles via the right and left bundle branches. These terminate distally as branching Purkinje fibres which penetrate and activate the ventricles. This ventricular activation (or depolarisation) sequence produces a QRS complex on the surface ECG and subsequent repolarisation gives rise to an electrocardiographic T wave. Pathophysiological processes may disrupt this sequence, giving rise to arrhythmia production.1,2
Arrhythmogenic Mechanisms
Abnormal Automaticity
The action potential of sinus and atrioventricular conducting tissue differs from that of the myocardium in that phase 4 of their action potentials are less stable and possess the property of spontaneous automaticity and consequent depolarisation. This is an important property that allows these tissues to assume the role of electrophysiological pacemaker dominance. However, in some circumstances, such as myocardial ischaemia or cardiostimulatory influences, regional levels of spontaneous automaticity can be abnormally accelerated, stimulating subsidiary pacing cells (such as those within the AV junction and ventricular Purkinje fibres) to override the normal sinus rate.3,4
Triggered Activity
Arrhythmias may occur through the occurrence of abnormal oscillations within the early and late repolarisation stages of the cardiac action potential that lead to the propagation of aberrant ‘triggered’ arrhythmic events. Such oscillations are classified as either ‘early after depolarisations’ that occur during phases 2 and 3 of the action potential or late after depolarisations, which occur during phase 4. Digitalis toxicity, ischaemia, hypokalaemia, hypomagnesaemia and elevated catecholamine levels are the more common causes of triggered activity.5 Excessive prolongation of the action potential duration enhances the risk of such triggered activity and as such these mechanisms are implicated in the development of certain subtypes of ventricular tachyarrhythmias, in particular torsade de pointes (refer to description later in this chapter).
Reentry
The most common cause of tachyarrhythmias is reentry, in which current can continue to circulate through the heart because of different rates of conduction and repolarisation in different areas of the heart (temporal dispersion). Slow conduction through a region of the heart may allow enough time for other tissues which have already been depolarised to recover, and then to be re-excited by the arrival of the slowly-conducting wavefront. Once this pattern of out-of-phase conduction and repolarisation is established, a current may continue to circulate back and forth between adjacent areas, or around a re-entry circuit. Each ‘lap’ of the circuit gives rise to another depolarisation (P wave or QRS complex).4,6 The ultimate rate of the tachycardia depends on the size of the circuit (micro versus macro reentry) and the conduction velocity around the circuit.
Arrhythmias and Arrhythmia Management
Arrhythmias may arise from myocardial or conduction system tissue, and may represent inappropriate excitation or depression of automaticity, altered refractoriness resulting in micro-reentry arrhythmias, or may involve reentry on a larger scale, as between the atria, AV node and/or ventricles.3
The clinical impact of tachyarrhythmias is highly variable and is influenced by the rate and duration of the arrhythmia, the site of origin (ventricular vs supraventricular), and the presence or absence of underlying cardiac disease. As a result, arrhythmias may require no treatment, at least in the short term, or at worst may present as cardiac arrest and require treatment according to advanced life support algorithms (as described in Chapter 24).
Arrhythmias of the Sinoatrial Node and Atria
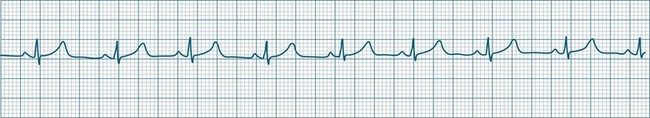
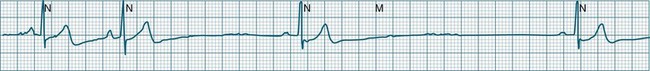
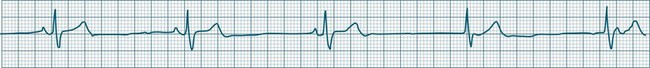
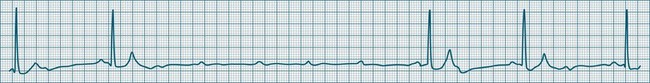
In health, the sinus node controls the heart rate according to metabolic demand, responding to autonomic, adrenal and other inputs, which vary according to exertion or other stressors. In response to needs, the sinus node discharge rate typically varies from as low as 50 beats/min to as high as 160 beats/min. In the conditioned heart (e.g. in athletes), this range extends perhaps down to as low as 40 beats/min, and to as high as 180 beats/min. Peak activity in the elite athlete may even achieve sinus rates of 200/min, though this represents the extreme end of the sinus rate. Sinus rhythm is illustrated in Figure 11.1.
Sinus Tachycardia
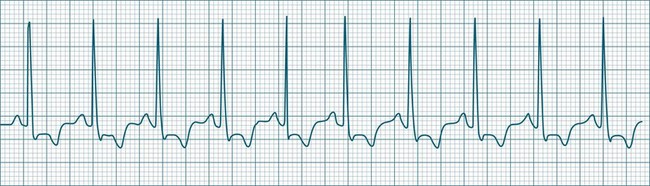
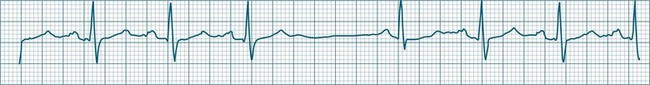
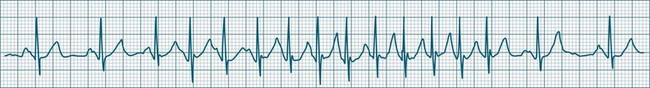
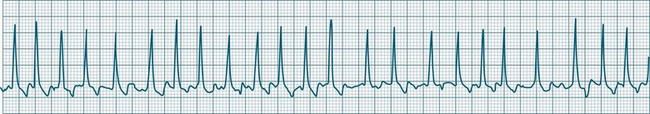
In adults, a sinus rate of greater than 100/min is termed sinus tachycardia and may occur with normal exertion7,8 (see Figure 11.2). When sinus tachycardia occurs in the patient at rest, reasons other than exertion must be sought and include compensatory responses to stress, hypotension, hypoxaemia, hypoglycaemia or pain, in which there is increased neurohormonal drive. Many drugs such as inotropes and sympathomimetics also accelerate the sinus rate. Sinus tachycardia should therefore be regarded as a response to a physiological stimulus rather than an arrhythmia arising from sinus node dysfunction. Treatment is directed at the trigger for the tachycardia, not the tachycardia itself. As sinus tachycardia may point to covert events such as internal bleeding or pulmonary embolism, there should be thorough investigation for unexplained, persistent sinus tachycardia.
Sinus Bradycardia
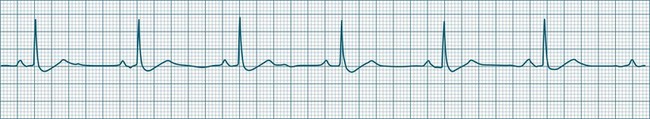
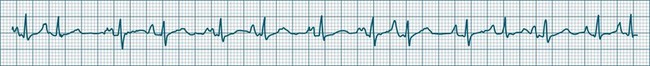
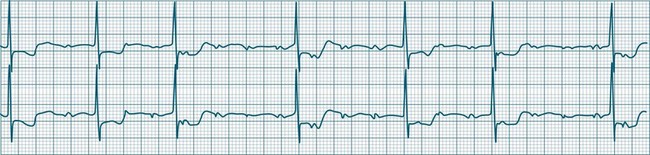
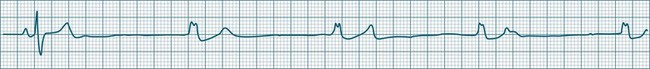
A sinus rate of less than 60 beats/min is termed sinus bradycardia7,8 (see Figure 11.3). In general terms the slower the rate, the more likely it is to produce symptoms related to low cardiac output. Slowing of the rate to less than 50/min is commonplace during sleep, especially in the athletic heart, but is otherwise uncommon. Bradycardia may accompany myocardial ischaemia (especially when due to right coronary artery disease), conduction system disease, hypoxaemia, and vagal stimulation (e.g. nausea, vomiting, or painful procedures). It also accompanies beta-blocker, antiarrhythmic or calcium channel blocker treatment.9 Treatment of sinus bradycardia reflects the treatment of AV block and is covered below under the management of atrioventricular block.
Sinus Arrhythmia
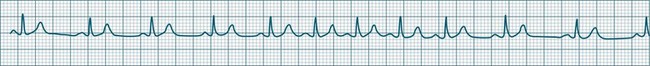
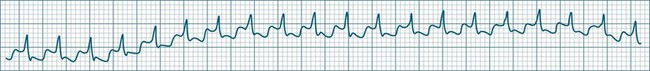
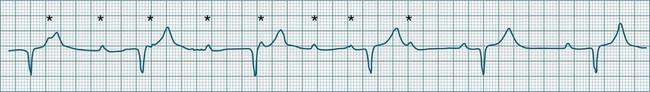
When the rhythm is clearly sinus in origin but is irregular, then the term sinus arrhythmia may be used (see Figure 11.4). Generally, a gradual rise and fall in rate can be appreciated in synchrony with respiration. The gradual rise and fall in rate is important: it distinguishes sinus arrhythmia from the abrupt prematurity with which atrial ectopic beats make their appearance, or the abrupt slowing of the sinus rate seen in sinus pause and sinus arrest. Sinus arrhythmia may accompany sinus node dysfunction but is seen also in the normal heart. Of itself, sinus arrhythmia does not require treatment.
Sinus Pause and Sinus Arrest
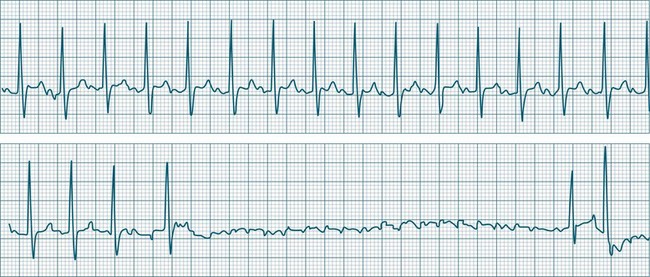
Abrupt interruption to the sinus discharge rate has spawned a variety of descriptive terms, based partly on physiology and partly on severity. Sinus pause is self-descriptive: during a period of sinus rhythm, there is a sudden pause during which the sinus node does not fire.9 The heart rate abruptly drops, during which time there may be bradycardic symptoms. Sinus arrest tends to be used as a descriptor when the sinus pause is longer rather than shorter (usually above 3 seconds) (see Figure 11.5). The longer the period of sinus arrest, the greater the likelihood of symptoms, and syncope is possible.9 Sinus pause may be indistinguishable from sinus exit block (in which there is sinus discharge that fails to excite the atria), as both result in missing P waves. The distinction is academic, however, as both arrhythmias arise from the same groups of causes, and are significant only when they cause symptomatic bradycardia. Pauses in which the P–P intervals spanning the pause are multiples of the pre-pause P-P interval favour the diagnosis of exit block (Figure 11.6).5 Recurrent syncopal pauses may require acute responses for symptomatic bradyarrhythmias (see AV block treatment below). If episodes continue, consideration should be given to permanent pacemaker implantation.
Arrhythmias of the Atria and Atrioventricular Node
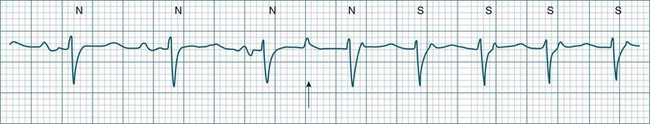
The term supraventricular tachycardia (SVT) is often used to group the tachyarrhythmias which arise from tissues above the ventricles. In its more common usage, SVT is thus an umbrella term, to include any of the tachyarrhythmias arising from the sinus node, the atrial tissue or the atrioventricular node.10 However, when a specific arrhythmia can be classified, the specific term is used rather than the more general term SVT. On occasion the electrocardiographic distinction between atrial flutter, atrial tachycardia and atrioventricular nodal reentry tachycardia may be difficult to make, and it may be useful in that context to use the more general term SVT. Supraventricular arrhythmias may occur as single-beat ectopics arising from atrial or junctional tissue, or runs of consecutive premature beats, and thus be termed supraventricular tachycardias. SVTs may be self-limiting (paroxysmal) or sustained (until treatment), recurrent or incessant (sustained despite treatment).
Atrial Ectopy
Impulses arising from atrial sites away from the sinus node (atrial foci) conduct through the atria in different patterns to sinus beats, and so give rise to P waves of different morphologies. These altered P waves define atrial ectopy, and their prematurity, or faster discharge rate, sees them more completely described as premature atrial beats. A characteristic P wave morphology cannot be provided, as ectopy may arise anywhere within the atria, causing upright, inverted or biphasic P waves. Ectopic P waves are often so premature that they become hidden within the preceding T wave. At such times evidence of their presence can be concluded only because they deform the T wave, and because premature QRS complexes of normal morphology follow, suggesting a supraventricular origin of those beats. Premature atrial beats most commonly conduct normally, although they may conduct aberrantly, or not at all, depending on their degree of prematurity and the state of AV nodal and intraventricular conduction (see Figure 11.7).
Atrial Tachycardia
A rapidly firing atrial focus or (more commonly) the presence of an atrial reentry circuit may give rise to a rapid rate, which is termed atrial tachycardia. Rates range from 140–230 beats/min and the rhythm is typically very regular.5 P waves may be difficult to identify, as they become hidden in T waves. At such times, the presence of narrow QRS complexes, confirming supraventricular conduction, aid diagnosis and discrimination from ventricular tachycardia. Distinction from other supraventricular arrhythmias may rely on the absence of characteristic features of other SVTs (e.g. the sawtooth baseline of flutter, the irregularity of fibrillation, or the pseudo-R waves and onset pattern of atrioventricular nodal reentry tachycardia). When the atrial rate exceeds the conduction capability of the AV node, varying degrees of AV block occur. Atrial tachycardia may be paroxysmal, sustained or incessant (see Figure 11.8). Symptoms vary and are partly dependent on the rate of the arrhythmia, and the presence or absence of myocardial dysfunction.
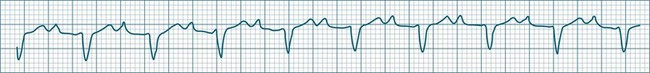
Multifocal Atrial Tachycardia
When multiple atrial sites participate in generating atrial ectopic beats at a rapid rate, the term multifocal atrial tachycardia is used (see Figure 11.9). The different foci produce P waves of varying morphology, and typically the strict regularity seen during atrial tachycardia is lost.9 Multifocal atrial tachycardia in particular complicates chronic obstructive pulmonary disease (COPD), as well as other pulmonary diseases as part of the cor pulmonale spectrum.11
AV Conduction During Supraventricular Tachyarrhythmias
The rapid atrial rates associated with some atrial arrhythmias exceed the conduction capability of the AV node, with the result that not all of the atrial impulses can be conducted (see Figures 11.10 and 11.11). This usually occurs when the atrial rate exceeds 200/min. Thus during atrial flutter, or rapid atrial tachycardia, it is common to see 2 : 1 block or greater. During atrial fibrillation the ventricular response rate rarely exceeds 170/min.
Atrial Flutter
Atrial flutter is a rapid, organised atrial tachyarrhythmia (see Figure 11.11). The atrial rate may be anywhere between 240 and 430/min, but most commonly the rate is close to 300/min.9 At these rates the atrial depolarisation waves (flutter waves) run together to produce the characteristic ECG feature of this arrhythmia: the so-called ‘sawtooth’ baseline, because of its resemblance to the teeth of a saw. This sawtooth baseline is generally best shown in the inferior leads. By contrast, in lead V1 the flutter waves usually appear more like discrete P waves, whilst in leads I and aVL, it may appear more like fibrillatory waves. The atrial rate of close to 300/min rarely conducts on a 1 : 1 basis to the ventricles. Rather 2 : 1, 3 : 1, 4 : 1 or variable levels of AV block intervene to limit the ventricular response rate, often to between 75 and 150/min.9 When the AV block is variable, beats at 3 : 1, 4 : 1 or other ratios are seen together in a single strip. When there is 2 : 1 block, the flutter waves are often concealed within the QRS and/or T wave, and so definite identification may be difficult (see Figure 11.12). At such times, the presence of a narrow QRS tachycardia at a fixed rate close to 150/min is particularly suggestive of atrial flutter with 2 : 1 block. The tendency for flutter waves to appear as discrete P waves in lead V1 may also be useful, as they may be more easily visualised in this lead. Vagal manoeuvres, or adenosine administration, may increase the degree of block and so reveal the flutter waves (Figure 11.12).7,8
Atrial Fibrillation
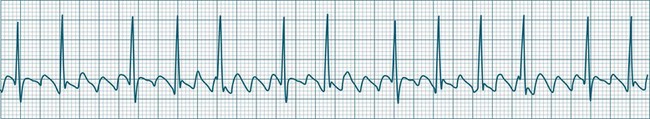
Atrial fibrillation is a chaotic atrial rhythm in which multiple separate foci either discharge rapidly or participate in reentry circuits, resulting in rapid and irregular depolarisations that are not able to gain complete control of the atria.7,9 Discrete P waves (representing the coordinated depolarisation of the atria) are therefore not seen; rather there is a continuous undulation of the ECG baseline (fibrillatory waves at a rate between 300 and 500/min), reflecting the continuous erratic electrical activity within the atria. This erratic, uncoordinated electrical activity results in uncoordinated contraction, and the atria can be seen not so much to contract but to quiver continuously. It is this quivering (fibrillatory) motion that gives atrial fibrillation its name.
The irregularity of the atrial rate results in an irregular arrival of impulses at the AV node and, as a result, conduction to the ventricles at irregular intervals.7 Thus, a hallmark of atrial fibrillation is the marked irregularity of the ventricular rhythm. The ventricular response rate to the rapid atrial rate is determined by the state of AV nodal conduction, and in patients with normal AV conduction is often in the range of 140–180/min (rapid or uncontrolled atrial fibrillation) (see Figure 11.13). Alternatively, when AV conduction is impaired, or limited by drug effect, slower ventricular rates are seen. When atrial fibrillation is accompanied by a ventricular rate less than 100/min, it may be termed slow (or controlled) atrial fibrillation. Atrial fibrillation is a common significant arrhythmia12 and, while not usually immediately life-threatening, it contributes significantly to morbidity, especially in patients with existing cardiac failure. The loss of organised atrial contraction (atrial kick) as well as rapid rates deprive the ventricles of adequate filling, and so hypotension and low cardiac output may result. Consequent pooling of blood in the atria enhances the risk of emboli formation and stroke. In addition, the incomplete atrial emptying results in congestion of first the atria and then the pulmonary circulation, and contributes to dyspnoea, increased work of breathing, and hypoxaemia. Patients with left ventricular failure rely more heavily on atrial kick, and so symptoms and the severity of their heart failure typically worsen during atrial fibrillation. At times, atrial fibrillation is debilitating in this group, and shock and/or acute pulmonary oedema may develop.
Antiarrhythmic therapy aims at reverting atrial fibrillation, or to limiting the ventricular rate (rate control) even if fibrillation is persistent.12 For patients with chronic atrial fibrillation in whom adequate rate control cannot be achieved pharmacologically, it is sometimes necessary to perform radiofrequency ablation of the AV node itself. Permanent pacemaker implantation is therefore also necessary.
Atrioventricular Nodal Reentry Tachycardia
Atrioventricular Nodal Reentry Tachycardia (AVNRT) is the most common type of paroxysmal supraventricular tachycardia (PSVT), accounting for greater than 50% of cases of PSVT.5 (Note that PSVT as used here does not include atrial flutter or fibrillation). AVNRT is more common in women (75% of cases), more often in younger than older patients, and in some individuals there is an identifiable link to stress, anxiety or stimulants. As the name suggests the arrhythmia arises because of reentry involving the AV node. Normally, atrial impulses reach the AV node via both slow and fast AV nodal pathways which link the atria to the AV node proper. The resultant PR interval is <0.20 sec. In AVNRT, the trigger mechanism is a premature atrial ectopic which is blocked by the fast pathway because of refractoriness. Conduction into the AV node and to the ventricles is still possible by the slow AV nodal pathway, but the resultant PR interval will be quite long (AV delay plus slow conduction into the AV node). Following this atrial ectopic with its long PR interval is the onset of the tachycardia.13
The tachycardia develops because the initiating impulse, the atrial ectopic, is delayed in reaching the AV node. Once it does reach the AV node it conducts to the ventricles, but also now finds the previously refractory fast pathway recovered and able to conduct retrogradely back to the atria. There is now a functional circuit for reentry between the atria and the AV node. Impulses conduct slowly into the AV node, lengthening the PR interval, but on reaching the AV node conduct just as quickly to atria as to the ventricles. As a result, the P waves appear at much the same time as the QRS.13 In some instances of AVNRT it is not possible to identify P waves at all because they are hidden within the QRS. Often, however, the P waves can be seen distorting the final part of the QRS complex, appearing as small R waves in V1 and small S waves in lead II. Because they are P waves rather than part of the QRS, the ECG appearance has been dubbed ‘pseudo R waves’ in V1 and ‘pseudo-S waves’ in lead II13 (Figure 11.14). AVNRT is typically regular, and most commonly at rates between 170 and 240/min but may be slower. The QRS is narrow unless there is concommitant bundle branch block. AVNRTs sometimes respond well to vagal manoeuvres, including coughing, bearing down, and carotid sinus massage. Adenosine may interrupt the arrhythmia, and other AV blocking drugs or antiarrhythmics may be necessary to prevent recurrence. Elective cardioversion is sometimes necessary, and if the arrhythmia is chronically troublesome, slow pathway ablation may be undertaken.5,13
Nursing Management of Atrial Arrhythmias
General symptoms of atrial tachyarrhythmias include: palpitations, dyspnoea/tachypnoea, fullness in the throat/neck, fatigue, lightheadedness, syncope, chest pain and angina symptoms and nausea and/or vomiting. Management of atrial tachyarrhythmias includes: (a) searching for and correction of the cause; (b) rate control limiting the ventricular response, even if the arrhythmias cannot be suppressed;14,15 (c) reversion of the arrhythmias by vagal manoeuvres, medication, cardioversion or overdrive pacing; (d) ablation;16 (e) prophylactic anticoagulation; and (f) prevention of recurrence using cardiac resynchronisation therapies such as biventricular pacing.17
Bradyarrhythmias and Atrioventricular Block
Bradycardia, a slowing of the ventricular rate to less than 60 beats/min, may occur in the form of slowing of the sinus node rate or failure of conduction at the level of the AV node. As the rate slows, escape rhythms should intervene, limiting the severity of the bradycardia. However, these may also fail, rendering the patient asystolic or with catastrophic bradycardia.18,19
Bradycardic Influences
Conduction system depression may occur with abnormal autonomic balance (increased vagal or decreased sympathetic tone), decreased endocrine stimulation (reduced catecholamine or thyroid hormone secretion), or from pathological influences such as conduction system disease, or congestive, ischaemic, valvular or cardiomyopathic heart diseases. Many biochemical and pharmacological factors cause conduction system depression with resultant bradycardia.18 The causes of bradycardia and AV block include:18
• drugs: virtually all antiarrhythmics, calcium channel or beta-blockers, and digitalis preparations may contribute to bradycardia and AV conduction disturbance to a greater or lesser extent
• decreased sympathetic activity, or blockade of neural transmission (e.g. spinal injury, anaesthetic or receptor blockade)
• increased parasympathetic activity: vagal stimu-lation such as nausea, vomiting, carotid sinus pressure, increased abdominal pressure, femoral manipulation.
In the absence of stimulation by the SA node, other tissues within the conduction system and myocardium can generate cardiac rhythms at rates slower than the normal sinus rate. Thus sinus node failure need not severely compromise the patient, as the inherent automaticity of the AV node can generate a (nodal) rhythm at a rate of 40–60 beats/min. Similarly, should the AV node fail and the ventricles receive no stimuli, there is an additional layer of protection, as the ventricles themselves can generate (ventricular) rhythms at rates of 20–40 beats/min.7
Junctional Escape Rhythms
This term describes the AV node response to bradycardia. When sinus bradycardia falls to a rate slower than the inherent automatic rate of the AV node, then the junctional tissues fire.7,9 Typical rates are 40–60/min but may be slower, as the cause of the primary bradycardia may also suppress the firing of escape foci. Intraventricular conduction usually follows the same pattern as had been present before junctional rhythm and so the QRS is unchanged from how it was previously, although occasionally aberrant ventricular conduction may occur, widening the QRS complex. P waves may or may not be evident and are often inverted because of retrograde conduction, as atrial activation spreads from the AV node and upwards through the atria. These P waves may at times be seen in advance of the QRS (at shorter than normal P–R intervals), within the ST segment, or may be hidden within the QRS complexes (see Figure 11.15).
Ventricular Escape Rhythms
When either the sinus or AV node fails, and stimulation of the ventricles does not occur, the ventricles can autoexcite themselves, usually at a rate of 20–40 beats/min (Figure 11.16). Symptoms of bradycardia commonly accompany these idioventricular rates, and acute rate restoration may be necessary. However, true cardiac arrest requiring cardiopulmonary resuscitation is less common, with the escape rhythm providing sufficient cardiac output to sustain vital functions in the short term. ECG features of idioventricular escape beats include:
• single ventricular ectopic beats occurring after a pause in the dominant rhythm, or as groups of beats at the slow escape rate
• QRS >0.12 sec, often notched, larger in amplitude and bizarre
• ST segment and T wave, often in the opposite direction to the major QRS direction.
When these beats occur at a rate of 20–40/min the rhythm is termed ventricular escape, or idioventricular rhythm. Under excitatory influences the ventricular pacemaker cells may increase their firing rate to between 60 and 100/min (accelerated idioventricular rhythm) or to faster than 100/min (ventricular tachycardia).20
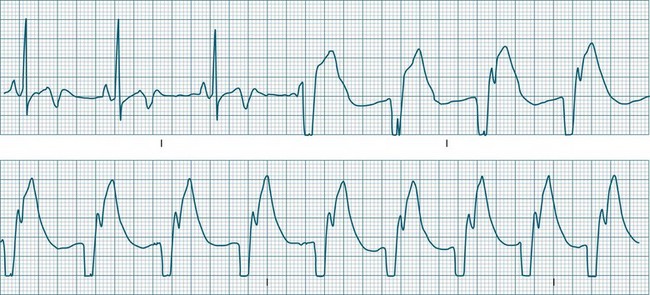
Accelerated Idioventricular Rhythm
Accelerated idioventricular rhythm (AIVR) has assumed a special place in cardiology because of its relatively common appearance during postinfarction reperfusion, thus often indicating successful revascularisation following PCI or thrombolytic therapy.20,21 It may therefore imply therapeutic success rather than mishap, and usually needs no treatment. The arrhythmia is commonly due to increased automaticity and as with other automaticity arrhythmias may show a ‘warm-up’ in rate, i.e. it may commence and then gradually accelerate and settle at a faster rate. This behaviour can be useful in differentiating arrhythmias from reentry which typically have an abrupt change in rate as their onset. When it occurs outside of the context of reperfusion, AIVR should be regarded as inappropriate ventricular excitation (Figure 11.17).
Atrioventricular Conduction Disturbances
Atrioventricular conduction disturbances make their appearance as delayed or blocked conduction from atria to ventricles, and thus appear as altered P–QRS (or P–R) relationships. The conventional classifications for AV block are based purely on the patterns of conduction. The classification as first-, second- and third-degree partially represents the severity of AV node or His-bundle dysfunction.7,9 AV block may complicate heart disease but is also seen commonly with drug therapy (e.g. digitalis, calcium channel blockers, beta-blockers and other antiarrhythmics).20 It may occur abruptly following vagal stimulation. When accompanying myocardial infarction, it is more likely to be transient following inferior infarction; whereas its appearance following anterior infarction is more likely to be permanent.
Degrees of Atrioventricular Block
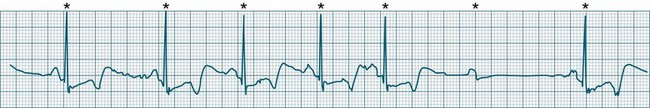
First-degree AV block
All atrial impulses are conducted to the ventricles but conduction occurs slowly, with a P-R-interval >0.20 sec. 1 : 1 AV conduction is maintained (see Figure 11.18).
Second-degree AV block
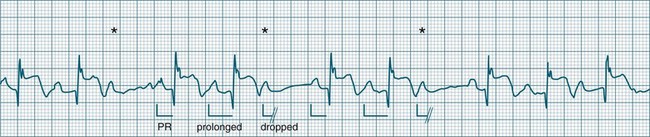
• Second-degree AV block type I (Wenckebach): A cyclical pattern of AV conduction is seen in which the conducted P waves show a progressive lengthening of the P–R interval until one fails altogether to be conducted (blocked, or dropped, P waves). Cycles begin with a normal or (often) prolonged P–R interval, which then extends over succeeding beats until there is a dropped beat. After the dropped beat the cycle recurs, commencing with a P–R interval equivalent to that commencing previous cycles63 (Figure 11.19). The frequency of dropped beats partially represents the severity of AV block. When, for example, every fifth P wave is not conducted, 5 : 4 conduction is said to be present. If AV conduction deteriorates further, more frequent P waves fail to be conducted (4 : 3, 3 : 2 conduction).
• Second-degree AV block Mobitz type II: Dropped beats (non-conducted P waves) are also present, but the conducted beats show a uniform P–R interval rather than any progressive lengthening9 (Figure 11.20). The dropping of beats may be regular, e.g. every fourth P wave (termed 4 : 1 block), progressing to 3 : 1, or even 2 : 1 block as AV nodal, or more commonly, His-Bundle conduction, worsens. Alternatively, the dropping of beats may be more irregular (variable block), with combinations of 2 : 1, 3 : 1, 4 : 1 or other levels of block evident in a given strip. The more frequent the dropped beats, the slower the ventricular rate and the greater the likelihood of symptoms. Second-degree Type II AV block is often associated with intraventricular conduction delay, with corresponding widening of QRS complexes. When this is seen it represents conduction impairment not just of the AV node but of intraventricular conduction as well. Progression to complete AV block is more common.9
A final form of second-degree block is ‘high-degree’ AV block, in which conducted P waves show a uniform P–R interval but, rather than single periodic dropped beats, multiple consecutive non-conducted P waves can be seen (Figure 11.21).
Third-degree (complete) AV block
None of the atrial impulses are conducted to the ventricles, resulting in a loss of any relationship between P waves and QRS complexes (AV dissociation). Usually a lower pacemaker assumes control of the ventricular rate, and this focus may be either junctional (narrow QRS, at a rate of 40–60/min) or ventricular (wide QRS, at a rate of 20–40/min) (Figure 11.22).9
Nursing Management During AV Block
AV block may be progressive in nature, and may worsen with advancing heart disease or after introduction, or dose modification of drugs that depress AV conduction.23,24 Thus monitoring should include P–R interval measurement, and where the P–R interval becomes prolonged there should be an increase in vigilance directed towards further prolongation or the development of dropped beats, to signify advancing AV block. Treatment of AV block and bradycardia includes immediate assessment of cardiovascular status or other symptoms, including chest pain, dyspnoea, conscious state and nausea. The cause should be identified and treated where possible. Patients need to be on rest in bed, provided with reassurance and oxygen by mask or nasal prongs. If the patient is hypotensive, IV fluids should be administered and the patient laid flat. Standardised protocols for bradycardia should be applied if the patient is symptomatic, and these usually include:18
• atropine sulphate 0.5–1.0 mg IV25
• isoprenaline hydrochloride in 20–40 mcg increments,26 with an infusion at 1–10 mcg/min
• transthoracic pacing (usually with sedation)
If the patient is pulseless or unconscious, standard advanced life support should be administered (see Chapter 24). Persistent or recurrent symptomatic bradycardia or AV block may require permanent pacemaker implantation.18,19
Ventricular Arrhythmias
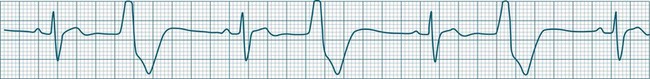
Ventricular ectopic rhythms may either occur as a response to slowing of the dominant cardiac rhythm (escape beats or escape rhythms) or may emerge at faster rates than the dominant rhythm (as premature ectopic beats, couplets, or ‘runs’ of ventricular tachycardia).9 Escape rhythms (occurring after a pause) should be regarded as physiological, as they protect against otherwise severe bradycardia (see Figure 11.16), whereas premature beats and rapid ventricular ectopic rhythms (occurring in advance of the dominant rhythm) occur when pathology gives rise to increased automaticity or reentry behaviour (Figure 11.23).7,9 Single ectopic beats may be benign occurrences, often seen in the absence of heart disease. However, their new appearance accompanying cardiac or systemic disease may precede the development of more serious arrhythmias, such as ventricular tachycardia or fibrillation, and thus warrant close monitoring. Ectopic beats, whether premature or late (escape), show characteristic features as follows:
• QRS complexes are wide (>0.12 sec) and of different morphology (large and bizarre in shape)27
• Notching of the QRS is common.
• ST segments and T waves are usually in the opposite direction to the major QRS deflection.
Ectopic beats may occur as single or coupled beats, or in runs of consecutive beats. Ventricular tachycardia is defined as greater than 3 consecutive ventricular beats occurring at a rate greater than 100/min.5
Causes of ventricular tachyarrhythmias include:3,8,28
• myocardial ischaemia, infarction
• cardiomyopathies/cardiac failure
• biochemistry: hypokalaemia, hypomagnesaemia, pH derangements
• adrenaline, isoprenaline, dobutamine, dopamine, levosimendan, atropine.
Patterns of Ectopy
Some patterns of ectopic frequency and morphology may warn of increasing risk for the development of serious arrhythmias such as ventricular tachycardia or fibrillation, and therefore earn a particular mention in monitoring. Historically, ectopic patterns have been graded according to their pre-emptive risk of serious arrhythmia development or 2-year mortality.29 Studies undertaken in 2003 and 2005 did however call into question the predictive status of certain ‘high risk’ ectopic patterns (such as ‘R on T’ ectopy), instead postulating that other factors such as a patient’s underlying left ventricular function and level of autonomic responsiveness may play a more significant role in the generation of life threatening ventricular tachyarrhythmias, independent of the prior presence or pattern of ectopy present.30,31 However, in the critical care context it is reasonable to respond to certain patterns (as shown in Box 11.1) by investigating and managing potential contributing causes. If the patient can be seen to be advancing through stages of increased arrhythmic complexity consideration for antiarrhythmic therapy should be given.
Box 11.1
Patterns suggesting higher risk of arrhythmia
Ventricular Tachycardia
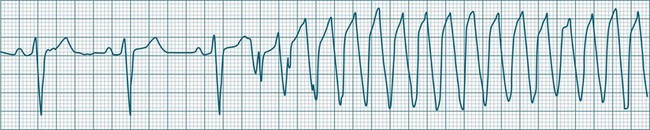
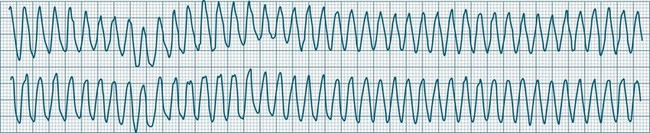
Ventricular tachycardia (VT) is described as a ‘run’ of three or more consecutive ventricular ectopic beats, at a rate greater than 100/min (Figure 11.24).12 The arrhythmia varies in its clinical impact, but when sustained is typically symptomatic with some degree of haemodynamic compromise. Ventricular tachycardia often presents as cardiac arrest, with the patient pulseless and unconscious, and is one of the major mechanisms of sudden cardiac death. The severity of symptoms depends partly on the rate (which may be 100–250/min), the duration of the arrhythmia, the presence of cardiac disease (ischaemic, congestive, hypertrophic, cardiomyopathic), and the presence of co-morbidities.9,32 When it develops, VT may be categorised as self-limiting (terminating without treatment), sustained for some period of time (minutes or longer), incessant (persisting until or despite treatment) or intermittent. Additional defining terminology includes monomorphic (all beats of the same morphology) or polymorphic (in which the rhythm conforms to the other features of VT but there is variability in the QRS shapes). ECG features of ventricular tachycardia:14,32,33
• Rate >100/min, rarely >240/min.
• Rhythm typically regular; there may be minor irregularity, especially on commencement and sometimes preceding self-termination.
• P waves may be absent. Atrial activity, whether dissociated or retrograde, is usually difficult to identify electrocardiographically.
• Morphology: QRS is wide (>0.12 sec). QRS often notched or bizarre in shape.
• Any axis is possible (normal axis, left or right axis deviation). An axis in the range of −90 to −180 degrees (‘no man’s land’) provides strong support for the diagnosis of ventricular tachycardia, as it implies the QRS originates at the apex and spreads through the ventricles upwards and to the right.
• ST segment and T wave displacement is in opposite direction to the major QRS direction.
If VT is not self-limiting, treatment depends on the severity of the symptoms. If the patient becomes pulseless and unconscious, advanced life support is initiated (see Chapter 24). If the patient is conscious and has a pulse, therapy can be undertaken more cautiously. Occasionally, robust coughing may revert VT in the cooperative patient. Antiarrhythmic therapy (at slower administration rates than during cardiac arrest) is usually undertaken first, along with biochemical normalisation. If unsuccessful, sedation and elective cardioversion may be necessary. Consideration for internal cardioverter defibrillator (ICD) implantation should be given to patients surviving ventricular tachycardia or fibrillation.34,35
Ventricular Flutter
This uncommon arrhythmia is most likely just a subset of ventricular tachycardia, but because of its rapid rate (at times up to 300/min or more) and the appearance of QRS complexes that are largely indistinguishable from the T waves, ventricular flutter has earned its own classification.32 An example is shown in Figure 11.25. The diagnostic separation from other types of VT is clinically unimportant, and treatment should follow normal guidelines for VT.
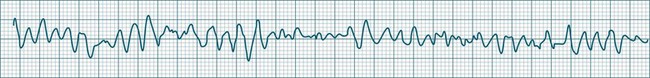
Ventricular Fibrillation
During ventricular fibrillation there is no recognisable QRS complex. Instead, there is an irregular and wholly disorganised undulation about the baseline.5,9 There are deflections, which at times approach rates of 300–500/min, but these are typically of low amplitude and none convincingly resemble QRS complexes (Figure 11.26). In the absence of organised QRS complexes the patient becomes immediately pulseless, and unconsciousness follows within seconds. Immediate defibrillation is required. If VF persists treatment occurs according to standing basic and advanced life support guidelines.
Polymorphic Ventricular Tachycardias
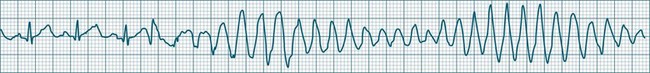
These forms of VT do not have a single QRS morphology. Rather, the QRS complexes during the rhythm vary from one shape to another, either alternating on a beat-to-beat basis or switching between groups of beats, with first one morphology and then another (bidirectional VT).9,32 The more common form of polymorphic VT is Torsades de Pointes (TdP), in which the QRS undergoes a gradual transition from one QRS pattern to another. The descriptive French term, literally ‘twisting of the points’, refers to the appearance of the ‘points’ (QRS direction), which is first positive and then negative, usually with an ill-defined transition between the two (Figure 11.27).28,36,37
ECG features of Torsades de Pointes are:28,36,37
• QRS polymorphic, with the transitions between polarity as described above.
• rate often very rapid, in the range of 300/min.
• regularity: the evident complexes are often regular, but particularly within the transition between QRS directions there may be irregularity.
• often self-limiting but recurrent.
• Q–T prolongation evident during normal rhythm (see Research vignette)
• often precipitated by R-on-T ectopic beats.
• commonly pause-dependant, with bradycardia or single beat pauses precipitating onset.
Because of the very rapid rate, syncope and cardiac arrest are common, and advanced life support practices required. A thorough search for possible causes of Q–T prolongation should be undertaken. Causes include: class Ia (procainamide, quinidine, disopyramide) or class III (amiodarone, sotalol) antiarrhythmics,5,9 erythromycin, antidepressants, hypocalcaemia, hypokalaemia and hypomagnesaemia.32 Congenital long Q–T syndromes also exist.36 Apart from the general ventricular arrhythmia management principles listed below, the treatment of TdP includes cessation of Q–T prolonging agents, a greater emphasis on IV magnesium, and the use of isoprenaline and/or pacing to shorten the Q–T interval and prevent bradycardia.38
Bradycardia in patients with long QT requires special mention as Torsades de Pointes is so often bradycardia, or pause, dependent. Pauses prolong the QT and favour ectopy which more easily find the T wave, triggering TdP. The role of pacing and isoprenaline are to both prevent pauses, and to shorten the QT interval.36,39
Management of Ventricular Arrhythmias
The emergency management algorithm for life-threatening ventricular arrhythmias is described in the chapter on resuscitation. In general terms, the management of ventricular arrhythmias should include the following:38
• a search for and correction of causes, including
• immediate CPR and cardioversion/defibrillation for pulseless, unconscious ventricular arrhythmias (cardiac arrest).38 In conscious patients, initial treatment is usually pharmacological, and, if necessary, cardioversion is applied under the influence of short-acting anaesthetics (e.g. propofol)
• heart failure management, which needs to be aggressive if contributory
• electrophysiological (EP) testing, which should be performed for serious arrhythmias to identify foci or pathways and confirm effectiveness of treatment41
• implantable cardioverter defibrillator therapy, which should be considered for all survivors of sudden cardiac death,34,35 especially those with low ejection fraction and recurrent sustained ventricular arrhythmias41
• where a myocardial scar can be confirmed as the arrhythmic focus, surgical resection may sometimes be undertaken.
Antiarrhythmic Medications
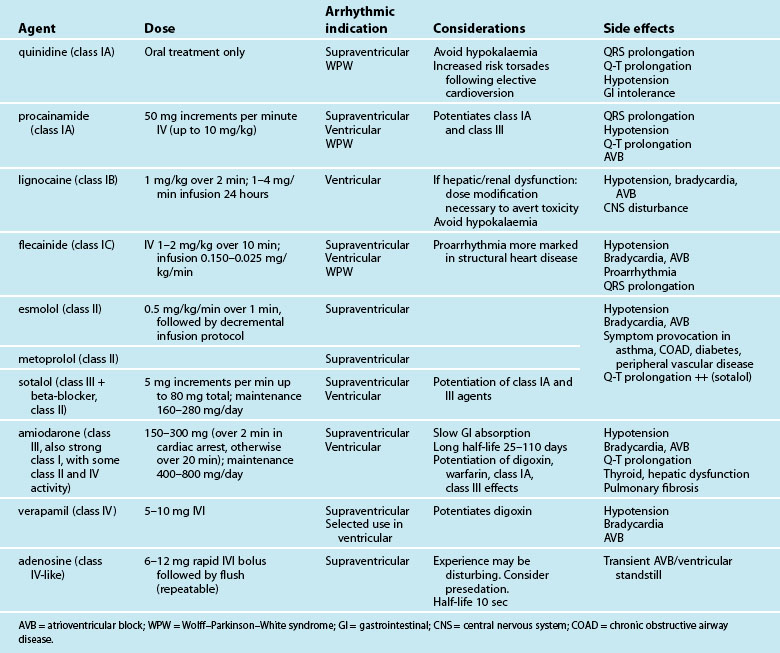
Antiarrhythmic drugs are classified partly on the basis of beta-receptor or membrane channel activity, and partly by their physiological effects on the cardiac action potential. This is well represented by the Vaughan Williams classification system (see Table 11.1).39 However, as action potential abnormalities cannot be expediently identified at the bedside, matching antiarrhythmic agents to cellular physiology cannot realistically be undertaken. Instead, antiarrhythmics are chosen partly on the basis of their known efficacy, by their suitablity to atrial or ventricular arrhythmias, and after consideration of side effects and contraindications to known comorbidities in a given patient.41,42
TABLE 11.1 Antiarrhythmic classifications43
| Class | Action | Drugs |
|---|---|---|
| IA | Sodium channel blockers: action potential prolongation | quinidine procainamide disopyramide |
| IB | Sodium channel blockers: accelerate repolarisation; shorten action potential duration | lignocaine mexiletine |
| IC | Potent sodium channel blockers: little effect on repolarisation | flecainide |
| II | Beta-blockers: depress automaticity (prolong phase 4); indirect prolongation phase 2 | metoprolol propanolol esmolol |
| III | Potassium (outward) channel blockers: prolong duration of action potential (prolonged repolarisation) | amiodarone sotalol (beta-blocker with class II actions) |
| IV | Calcium channel blockers | verapamil diltiazem |
Table 11.2 depicts the classification of the major acute antiarrhythmics in use in Australia and New Zealand, along with doses, arrhythmic indications, precautions and side effects. Class I agents all slow phase 1 (depolarisation) and so may slow down conduction and prolong the QRS. The subgroups of class I agents denote strength (A = weakest, C = strongest) and affect repolarisation, with class IA (prolonging), IB (shortening) and IC (not affecting) repolarisation duration. The class II agents (beta-blockers) depress automaticity, slowing the heart rate and prolonging the action potential. The class III agents notably prolong repolarisation, action potential duration and the Q–T interval. Class IV agents slow inward calcium channel flux, decreasing automaticity and prolonging the action potential.37
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree