C
Calcitonin
Also called: (CT); Thyrocalcitonin
Basics the nurse needs to know
To assess familial medullary cancer in relatives of patients with the cancer, a provocation test (also called a stimulation test) may be performed. Calcium chloride is given intravenously over 10 minutes, or pentagastrin is given intravenously over 5 to 10 minutes. Patients with medullary cancer will respond to these stimulants with excessive secretion of calcitonin.
How the test is done
A venipuncture is performed. Calcitonin is measured using radioimmunoassay (RIA).
Calcium, total and ionized, serum
Also called: Total calcium (CA); Ionized calcium (Ca1)
Basics the nurse needs to know
Decreased values
REFERENCE VALUES
Premature: 6.2-11.2 mg/dL or SI: 1.55-2.75 mmol/L
Infant (0-10 days): 7.6-10.4 mg/dL or SI: 1.90-2.60 mmol/L
(10 days to 24 months): 9.0-11.0 mg/dL or SI: 2.25-2.75 mmol/L
Child (24 mo-12 yr): 8.8-10.8 mg/dL or SI: 2.20-2.70 mmol/L
(12 – 18 yr): 8.4-10.2 mg/dL or SI: 2.10-2.55 mmol/L
Adult (18-60 yr): 8.6-10.0 mg/dL or SI: 2.15-2.50 mmol/L
Whole blood Adult (18-60 yr): 4.6-5.08 mg/dL or SI: 1.15-1.27 mmol/L
(60-90 yr): 4.64-5.16 mg/dL or SI: 1.12-1.32 mmol/L
Serum neonate, 24 hr: 4.40-5.44 mg/dL or SI: 1.10-1.36 mmol/L
Youth: 4.80-5.52 mg/dL or SI: 1.20-1.38 mmol/L
Adult: 4.64 mg/dL or SI: 1.16-1.32 mmol/L
Urine neonate, 24 hr: 4.20-5.48 mg/dL or SI: 1.05-1.37 mmol/L
Interfering factors
NURSING CARE
Nursing actions are similar to those used in other capillary puncture or venipuncture procedures (see Chapter 2), with the following additional measures.
Pretest
Posttest
Hypocalcemia.
The very low serum calcium can induce depression or psychosis, laryngeal stridor, tetany, convulsions, hypotension, and a weak, thready pulse. The patient will have hyperactive reflexes and positive Trousseau’s and Chvostek’s signs (Table 6). A severe decrease to 6 mg/dL (SI: 1.5 mmol/L) or less can be life threatening.
Table 6 Nursing Assessment for Hypocalcemia
| Test | Method | Positive result |
| Trousseau’s sign | Apply a blood pressure cuff to the arm. Inflate the cuff to 10 mm above the patient’s systolic pressure for 3 minutes. Observe the hand. Then deflate the cuff. | When the serum calcium level is low, the patient’s hand will develop a carpal spasm. The first three fingers extend rigidly. The fourth and fifth fingers flex and curl toward the palm of the hand. |
| Chvostek’s sign | Use the tip of the index or middle finger to tap just beneath the cheekbone (zygoma) and towards the ear. | When the serum calcium level is low, the same side of the face will demonstrate repeated tics or involuntary spasms of the facial muscles. |
Hypercalcemia.
The very high serum calcium level can induce polyuria, anorexia, nausea, tachycardia in an early stage or bradycardia in a later stage, muscle weakness, lethargy, and absent deep tendon reflexes. A severe elevation of 14 mg/dL (SI: 3.5 mmol/L) or more is likely to induce coma and can cause death from a cardiac arrest.
Calculus analysis
Also called: Kidney Stone Analysis; Renal Calculus Analysis
Basics the nurse needs to know
Struvite stones are sometimes called infection stones because of their association with chronic urinary tract infection. It is not known whether the stone causes the infection to occur or the infection causes the stone to form. These pale stones are usually large and soft. They are also called a staghorn calculus because of their characteristic shape. The chemical composition of struvite stones is magnesium ammonium phosphate and carbonate apatite. Struvite stones are sometimes called phosphate stones based on their chemical composition. The pH of the urine is often >6.0.
Significance of test results
NURSING CARE
Cancer antigen 125
Interfering factors
NURSING CARE
Nursing actions are similar to those used in other capillary puncture or venipuncture procedures (see Chapter 2), with the following additional measures.
Pretest
Posttest
Capnogram
Purpose of the test
Monitoring exhaled carbon dioxide (CO2) permits continuous evaluation of alveolar ventilation, reducing the number of ABG determinations needed. ETco2 may be used to evaluate ventilator changes and weaning parameters from mechanical ventilation. It will confirm endotracheal intubation because no capnographic waveform will occur if the tube is in the esophagus.
Basics the nurse needs to know
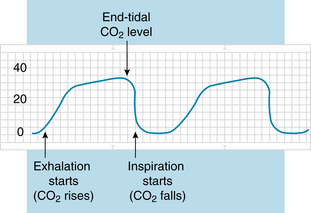
Capnography provides a CO2 waveform, which visualizes CO2 elimination patterns during exhalation and a total percentage of CO2 exhaled per breath. CO2 is measured at the end of exhalation because at this point the exhaled CO2 approximates arterial CO2 levels. With normal perfusion of the lungs, arterial CO2 will be a few millimeters higher (5 mm Hg) than end-tidal CO2 (ETco2). When perfusion is not adequate, this assumption cannot be made. Figure 28 shows a typical tracing of a capnogram.
How the test is done
For endotracheal tube placement, disposable colorimetric devices are available for single use.
Carbohydrate antigen 19-9
Also called: CA 19-9; Cancer Antigen CA19-9
Basics the nurse needs to know
Benign conditions of the pancreas, liver, and gall bladder also can cause an elevation of CA 19-9. In benign disease, the elevation is much lower than the dramatically high elevations associated with cancer. This test has a lack of specificity because it cannot distinguish between benign and malignant causes of hepatobiliary and pancreatic disease and various other diseases. Thus, because of false-positive results in benign conditions, this tumor marker does not have a clear role in the management of cancer patients (Marrelli, Caruso & Pedrazzani, 2009).
Interfering factors
NURSING CARE
Nursing actions are similar to those used in other venipuncture procedures (see Chapter 2), with the following additional measures.
Posttest
Carbon dioxide, total
Also called: tCO2,; TCO2; CO2 Content
Basics the nurse needs to know
Elevated values
Hypercapnia is associated with respiratory acidosis, CO2 retention, and metabolic alkalosis.
Decreased values
REFERENCE VALUES
Whole blood (venous), adult: 22-26 mEq/L or SI: 22-26 mmol/L
Whole blood (arterial), adult: 19-24 mEq/L or SI: 19-24 mmol/L
Serum, adult: 23-29 mEq/L or SI: 23-29 mmol/L
>60 yr: 23-31 mEq/L or SI: 23-31 mmol/L
Capillary (plasma), Newborn: 13-22 mEq/L or SI: 13-22 mmol/L
Infant: 20-28 mEq/L or SI: 20-28 mmol/L
Child: 20-28 mEq/L or SI: 20-28 mmol/L
Adult: 22-28 mEq/L or SI: 22-28 mmol/L
Carboxyhemoglobin
Basics the nurse needs to know
The hemoglobin molecule of a red blood cell has four receptor sites that will bind with oxygen molecules for transport of the oxygen to cells. When carbon monoxide is present in the blood, the hemoglobin has a powerful affinity to quickly attach the carbon monoxide instead of oxygen. The combination of carbon monoxide and hemoglobin form a compound called carboxyhemoglobin that cannot transport oxygen. As carboxyhemoglobin accumulates in the blood, tissue hypoxia begins to develop. In addition, the increasing accumulations of carboxyhemoglobin cause a shift of the hemoglobin-oxygen dissociation curve to the left, adding to the anoxia.
In laboratory measurements, the amount of carboxyhemoglobin in the blood is expressed as the percentage of hemoglobin that is saturated with carbon monoxide or the fraction of the whole that is saturated. As the amount of carboxyhemoglobin increases to 20% to 30% of hemoglobin saturation, symptoms of carbon monoxide poisoning appear. In children, lower concentrations may be toxic (McPherson & Pincus, 2007).
REFERENCE VALUES
Nonsmokers: 0.5%-1.5% Hb saturation or 0.005-0.015 Fraction of Hb saturation
Smokers (1-2 packs per day): 4%-5% Hb saturation or 0.04-0.05 Fraction of Hb saturation
Toxic: >20% Hb saturation or >0.20 Fraction of Hb saturation
Lethal: >50% Hb saturation or >0.50 Fraction of Hb saturation
Interfering factors
NURSING CARE
Nursing actions are similar to those used in other venipuncture procedures (see Chapter 2), with the following additional measures.
Posttest
 Nursing responses to critical values
Nursing responses to critical values
The patient may already have a toxic level of carbon monoxide poisoning at the time of admission for emergency treatment. The nurse assesses for symptoms including headache, nausea, dizziness, mental confusion, and elevations of the pulse, blood pressure, and respiratory rate. Late signs are the presence of the characteristic cherry red skin, loss of consciousness, hypotension, coma, seizures, and cardiac arrest (Goldstein, 2008). The nurse prepares to administer prescribed 100% pure oxygen via a tight-fitting, nonrebreather mask. Other emergency measures may be needed, such as intubation and cardiac/respiratory support.