Palpable lumps—date noted; affected by menstruation; changes noted since detection.
Nipple discharge—date of onset, color, unilateral or bilateral, spontaneous or provoked.
Pain or tenderness—localized or diffuse, cyclic or constant, unilateral or bilateral.
Date of last mammogram and result.
Patient’s practice of breast self-examination (BSE).
Age.
Past medical-surgical history; injuries; bleeding tendencies.
Medications, including current or prior use of hormonal contraceptives and hormones, over-the-counter (OTC) products, vitamins, and herbal supplements.
Menarche.
Date of last menses.
Pregnancies, miscarriages, abortions, deliveries.
Lactation history.
Prior breast history, including previous history of irradiation involving breast region.
Family history of breast cancer.
 Evidence Base
Evidence Base
Begin annual mammography at age 40. Annual clinical breast examination should be performed prior to mammography.
For women in their 20s and 30s, it is recommended that clinical breast examination be part of a periodic health examination, preferably at least every 3 years. Asymptomatic
women age 40 and older should continue to receive a clinical breast examination as part of a periodic health examination, preferably annually.
Beginning in their 20s, women should be told about the benefits and limitations of BSE. The importance of prompt reporting of any new breast symptoms to a health care professional should be emphasized. Women who choose to do BSE should receive instruction and have their technique reviewed on the occasion of a periodic health examination.
To detect abnormalities in the breasts
To teach a woman how to perform breast self-examination
Good lighting and a private, warm setting
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Screening decisions in older women should be individualized by considering the potential benefits and risks of mammography in the context of current health status and estimated life expectancy. As long as a woman is in reasonably good health and would be a candidate for treatment, she should continue to be screened with mammography.
 GERONTOLOGIC ALERT
GERONTOLOGIC ALERT Evidence Base
Evidence Base
Women at increased risk for breast cancer might benefit from additional screening strategies beyond those offered to women of average risk, such as earlier initiation of screening, shorter screening intervals, or the addition of screening modalities other than mammography and physical examination, such as ultrasound or magnetic resonance imaging (MRI).
Screening MRI is recommended for women with an approximately 20% to 25% or greater lifetime risk of developing breast cancer, including women with a strong family history of breast or ovarian cancer and women who were treated for Hodgkin’s disease. Other women at high risk include those over the age of 35 with a 5-year risk of invasive breast cancer >1.7% risk per the Gail model, women with a history of LCIS and atypical hyperplasia, and those with a prior history of breast cancer. Annual MRI should be considered in these women. The Gail model is available on the National Cancer Institute website at www.cancer.gov or at www.breastcancerprevention.com.
Wash nipple area with water and pat dry before obtaining specimen if crusting of drainage is present.
Gently milk breast or ask woman to express fluid to obtain a large drop on nipple.
Carefully touch slide to drop and draw slide across nipple to obtain smear. Spray with fixative or drop into container with fixative.
Inform patient of results promptly to reduce anxiety and explain that other tests may be needed.
Estrogen and progesterone receptors identify patients most likely to benefit from hormonal forms of therapy. Approximately 75% are estrogen receptor positive. A negative result is associated with a less favorable prognosis.
HER2—human epidermal growth factor receptor that has been demonstrated in 15% to 30% of breast cancers. Found by many investigators to be associated with poorer survival, regardless of clinical stage. May affect treatment decisions.
Histologic grade is reported using an Elston-Ellis modification of the Scarf-Bloom Richardson Scale. It is a combination of nuclear grade, mitotic rate, and tubule formation with scores given for each. A low score equates to a low grade (grade I), and a higher score to a higher grade (grade III). In general, high-grade tumors are more likely to recur when compared to low-grade tumors.
Multiparameter gene assays (eg, Oncotype DX and MammaPrint) quantify the likelihood of distant breast cancer recurrence and measure how chemotherapy may assist with planning treatment. The OncotypeDX assay is included in the National Comprehensive Cancer Network guideline treatment decision pathway. A similar assay for ductal carcinoma in situ (DCIS) is under investigation. Breast cancer diagnostic and treatment guidelines are available at www.nccn.org.
Cancer subtyping—as determined by gene expression profiling—is currently under investigation.
Pathologists may use other special stains to aid in diagnosis.
Increased values on liver function tests may indicate possible liver metastasis.
Increased calcium and alkaline phosphatase levels may indicate possible bony metastasis.
Additional metastatic workup may include chest x-ray, bone scan, computed tomography (CT) scan, and positron emission tomography (PET) scan.
Biological markers (ie, CA15-3 and CA27.29) may be used for monitoring patients with metastatic disease in conjunction with diagnostic imaging, history, and physical examination. Present data are insufficient to recommend their use alone for screening, diagnosis, or staging. However, they may be used to indicate treatment failure.
Low-dose x-ray of breast used to screen for breast abnormalities or may be used when a lump is found on physical examination. Can detect patients with clustered microcalcifications.
Compression of the breast is used to reduce the amount of radiation absorbed by the breast tissue and separate overlapping tissue.
Two views are taken routinely: craniocaudal and mediolateral; other views are done as necessary.
Best performed at a facility that is accredited by the American College of Radiology. The machines and staff at these facilities have met specific criteria. Computer-aided detection has been developed to aid radiologists in detecting abnormalities. Assessment categories have been developed to describe the results and provide follow-up recommendations.
Category 1 is a negative result (normal mammogram) with nothing on which to comment.
Category 2 is a normal mammogram but there is a benign finding on which to comment.
Category 3 is probably benign, but short interval follow-up may be recommended to determine stability of the finding.
Category 4 describes a suspicious abnormality and biopsy should be considered.
Category 5 is highly suggestive of malignancy requiring appropriate action.
Category 6 describes a known, biopsy-proven malignancy requiring appropriate action.
Mammography is not routinely done if a woman is pregnant.
The breasts of young women tend to be extremely dense and are poorly suited to mammography.
False-negative results occur even in the best facilities; figure may reach 10%.
Both screen film and digital mammography use x-rays to obtain images. With digital mammography, a film image is replaced with an electronic image similar to digital photography. For younger women under age 50, women with radiographically dense breasts, and premenopausal and perimenopausal women, digital mammography is more accurate than film mammography. For all other women, there is no significant advantage to using digital mammography.
 Evidence Base
Evidence Base
Recommend regular screening based on established guidelines (see page 884). Tell patients that routine screening mammography has been shown to reduce mortality from breast cancer. Procedure takes approximately 15 minutes.
Remind patients not to apply deodorant, cream, or powder to breast, nipple, or underarm areas on examination day.
Advise that some discomfort may be felt from compressing the breast.
Patients should have an opportunity to become informed about the benefits, limitations, and potential harms associated with regular screening. Overdiagnosis of clinically insignificant disease is possible. Benefits are thought to outweigh the exposure to low dose of radiation.
Alert patient that extra views do not imply that the patient has breast cancer.
 NURSING ALERT
NURSING ALERT
Uses high-frequency sound waves to get an image of the breast.
Helps determine if a lump is a cyst or a solid mass.
May be used if patient is pregnant or is younger than age 35.
Advise that this test is painless and noninvasive.
No preparation is necessary.
A contrast mammogram is obtained by injection of water-soluble contrast medium into a duct for patient with persistent bloody nipple discharge. It is a time-consuming procedure that is not routinely used.
It may outline an intraductal papilloma.
Its ability to differentiate benign from malignant lesions is limited.
Other tests such as tomosynthesis (3D mammography) and thermography may be useful in some women; however, these are not often used.
Produces images from the combination of a magnetic field, radio waves, and computer processing.
May be used in newly diagnosed breast cancer patients for presurgical planning. May help in determining extent of disease, multifocality, and unsuspected disease in the contralateral breast.
Useful in high-risk women, those with dense breasts, and in those with silicone implants.
Because MRI is less accessible and more expensive than mammography, it is not useful for generalized screening. There may be increased false-positive results. MRI-guided biopsy is not widely available.
See page 206 for description of MRI.
Uses a thin needle and syringe to collect tissue or to drain lump after using a local anesthetic. If it is a cyst, removing the fluid will collapse it; no other treatment may be needed. Ultrasound may be used to locate a nonpalpable cyst.
Normal cyst fluid appears straw-colored or greenish. Fluid should be sent for cytology if it appears suspicious (clear or bloody); otherwise, it is discarded.
This office procedure uses local anesthetic with results usually within 24 hours.
It has limited sensitivity, possibly because of insufficient acquisition of cytologic material.
Inform patient of small risk of hematoma and infection.
Adhesive bandage applied after procedure; usually no discomfort.
Solid lesions may warrant an excisional biopsy.
Office procedure uses local anesthetic and removes a small piece of breast tissue using a needle with a special cutting edge.
For palpable lesions with a high suspicion of malignancy. May provide a tissue diagnosis quickly—usually approximately 24 hours—without doing an excisional biopsy to plan definitive surgery.
Ultrasound guidance may be used for nonpalpable lesions.
Inform patient of small risk of hematoma and infection.
Tell patient that several passes may be necessary to obtain specimen, with minor discomfort.
Pressure dressing applied after procedure.
Recommend use of acetaminophen or ibuprofen for postprocedure discomfort—usually minimal, if any.
An x-ray-guided method for localizing and sampling nonpalpable lesions detected on mammography with 90% to 95% sensitivity in detecting breast cancer.
Performed as an outpatient procedure with the patient lying prone on a special table using an automated biopsy gun with a vacuum system to draw tissue into a sampling chamber and rotate cutter to excise tissue.
After local anesthetic is administered, a needle is placed in the lesion with confirmation of its position on stereotactic x-ray views. Multiple samples are taken from different portions of the lesion. Allows harvesting of larger quantities of tissue with a single needle insertion. A tiny clip may be left in place at the end of the procedure to mark the area.
The procedure is quicker and less expensive than mammographically guided needle localization followed by surgical excisional biopsy and is an alternative to surgical excisional biopsy to make a diagnosis.
Inform patient that it is a 1-hour outpatient procedure that requires no special preparation.
The patient should dress comfortably and will need to remain still during the procedure.
Complications may include minor bleeding, hematoma, and infection.
Explain that nonspecific, suspicious, or atypical findings may result in proceeding to excisional biopsy.
Remind patient that area in question will not be removed, only sampled.
Note: Many nonpalpable abnormalities that require breast biopsy are being identified because of increased use of screening mammography.
Surgical removal of a palpable or nonpalpable lesion. A frozen section may be done for immediate tissue diagnosis.
Excisional biopsy or lumpectomy entails entire removal of a mass; incisional biopsy entails partial removal of a mass.
This outpatient procedure may be performed under local or general anesthesia.
Curvilinear incision is usually made directly over the mass, which is excised en bloc including a 1-cm grossly free margin of tissue.
Pressure dressing is placed, which can be removed in 24 to 48 hours.
Inform patient to watch for bleeding, hematoma, and signs of infection.
Recommend analgesics for discomfort and a support bra for comfort.
May take several days to get results, a stressful time for patients.
Performed when there is a nonpalpable mammographic finding.
Mammogram is used as a guide for placing a needle at the site of the breast change after injecting some local anesthetic.
A wire may be left in place for the surgeon and dye may be injected to mark the site.
Excisional biopsy is then done (see above) removing the area around the tip of the wire.
A diagnostic surgical procedure utilizing selective lymph node sampling.
Pathologic study of the first (sentinel) axillary lymph node to receive drainage from a tumor; predicts the status of the remainder of the lymph nodes in the axilla.
Localization accomplished by injection of a blue dye and/or radioactive particles around a tumor to identify lymph nodes with afferent drainage.
The excised sentinel lymph nodes are subjected to routine pathologic examination and, possibly, immunohistochemical staining to detect micrometastasis.
The status of the sentinel lymph node is used to determine whether to proceed with full axillary dissection and/or determine treatment modalities.
If the sentinel lymph node tests negative, no further axillary surgery is indicated.
If test result is positive, further axillary dissection may be needed. There is currently debate regarding optimal treatment for node-positive disease in women undergoing breastconserving surgery and having fewer than three involved lymph nodes. The omission of completion axillary lymph node dissection in selected patents has increased with no difference in overall survival. There are no clear-cut guidelines at this time.
This is a reliable procedure, which is usually performed at the same time as definitive surgery—in conjunction with a breast-preserving procedure or mastectomy.
There is less morbidity and cost than with axillary dissection.
Examine breasts once per month, just after the menstrual period because breasts are less engorged and a tumor is easier to detect, and at regular monthly intervals after the cessation of menses.
Compare findings with the opposite breast.
Remind patient that 90% of breast lumps are not cancerous.
Do not neglect men when teaching BSE—1% of breast cancers occur in men.
It is acceptable for women to choose not to do BSE or to do BSE irregularly.
 NURSING ALERT
NURSING ALERT
Tenderness—gentle self-examination may be more effective and less painful than examination by someone else.
Cystic breasts—recommend professional examination annually, and instruct patient to compare changes in breasts from one month to the next.
Large, pendulous breasts—encourage woman to support her breast with her hand to palpate thoroughly; lying down may help to flatten breasts.
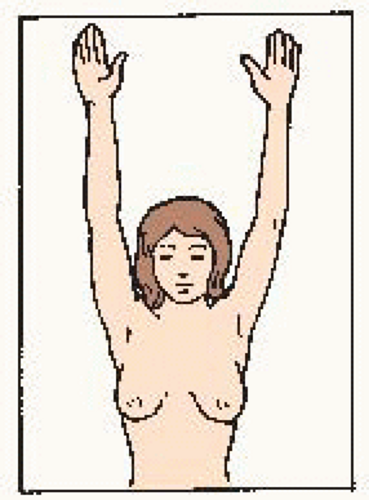
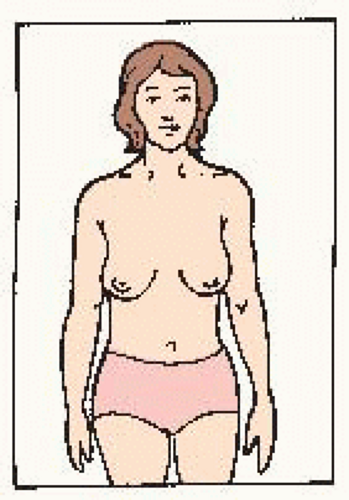 Hands at side. Compare for symmetry. Look for changes in: • shape • color Check for: • puckering • dimpling • skin changes • nipple changes |
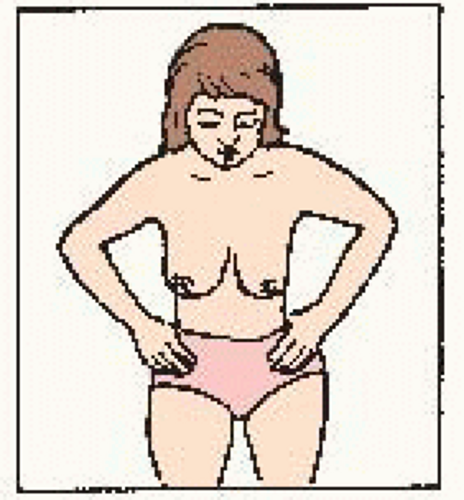 Hands on hips, press down, bend forward. Check for: • symmetry • nipple direction • general appearance |
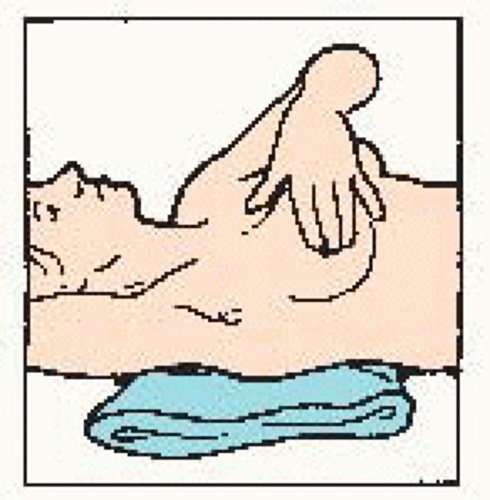
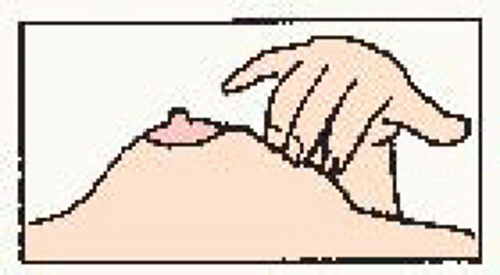
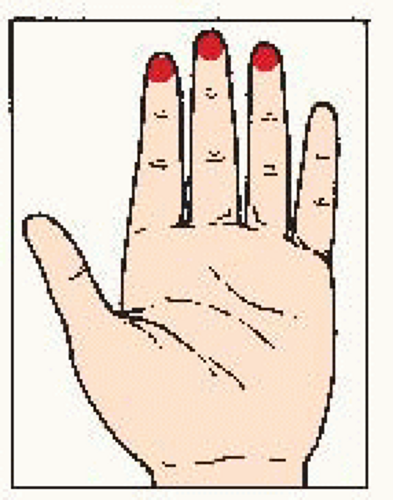 Use the pads of the three middle fingers of the left hand. Hold hand in bowed position. Move fingers in dime-size circles. |
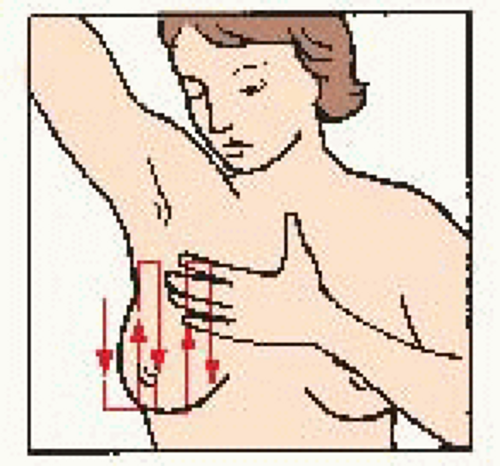
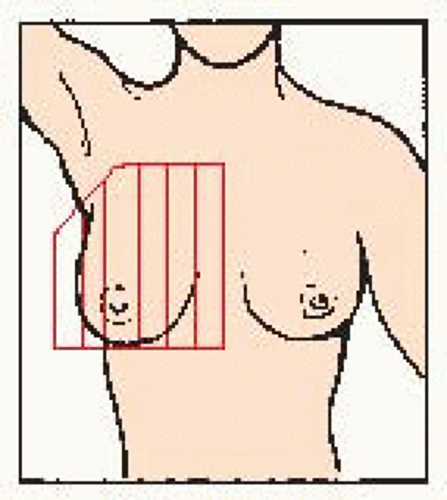 Examine area from: • underarm to lower bra line • across to breast bone • up to collar bone • back to armpit |
 Evidence Base
Evidence Base
Teaching BSE to women in the community—schools, churches, women’s groups.
Reinforcing that early detection is associated with decreased mortality.
Helping patients and families establish and maintain support networks.
Tailoring patient education messages to patients of different cultures.
Knowing the resources available and making people aware of them.
National Cancer Institute—answers questions and provides booklets about cancer. Call 800-4-CANCER or go to www.cancer.gov.
American Cancer Society—offers many services to patients and their families. Call 800-ACS-2345 for local chapter or go to www.cancer.org.
Table 23-1 Types of Surgery for Breast Cancer | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 GERONTOLOGIC ALERT Normal breast changes in older patients include drooping, flaccid breasts caused by decreased subcutaneous tissue from decreased estrogen levels. Nipple size and erection are also reduced.
GERONTOLOGIC ALERT Normal breast changes in older patients include drooping, flaccid breasts caused by decreased subcutaneous tissue from decreased estrogen levels. Nipple size and erection are also reduced. Evidence Base American Cancer Society. (2012). Can breast cancer be found early? Available: www.cancer. org/cancer/breastcancer/detailedguide/breast-cancer-detection.
Evidence Base American Cancer Society. (2012). Can breast cancer be found early? Available: www.cancer. org/cancer/breastcancer/detailedguide/breast-cancer-detection.