Bleeding in Pregnancy
Mary Ellen Burke Sosa
SIGNIFICANCE AND INCIDENCE
Hemorrhagic complications during pregnancy are a significant causative factor of adverse maternal-fetal outcomes. Major blood loss predisposes the woman to an increased risk of hypovolemia, anemia, infection, preterm labor/birth, and maternal death. Although bleeding can cause considerable problems for the mother, the fetus is especially in jeopardy because significant maternal blood loss can result in negative alterations in maternal hemodynamic status and decreased oxygencarrying capacity. When bleeding decreases blood flow to the placenta, maternal-fetal gas exchange is reduced and the fetus is at risk for progressive physiologic deterioration (e.g., hypoxemia, hypoxia, asphyxia, and death). This risk is directly related to the amount and duration of blood loss.
Hemorrhage during pregnancy is one of the leading causes of maternal death in the United States, along with embolism and hypertensive disorders (Berg, Callaghan, Syverson, & Henderson, 2010; Paxton & Wardlaw, 2011; The Joint Commission [TJC], 2010). According to the most recent data available from the Centers for Disease Control and Prevention (Berg et al., 2010), the aggregate pregnancy-related mortality ratio for the 8-year period from 1998 to 2005 was 14.5 per 100,000 live births, which is higher than any period in the previous 20 years of the Pregnancy Mortality Surveillance System. African American women continued to have a threefold to fourfold higher risk of pregnancy-related death (Berg et al., 2010), which may reflect social, economic, and cultural barriers to healthcare (Mahlmeister, 2010).The proportion of deaths attributable to hemorrhage and hypertensive disorders declined from previous years, whereas the proportion from medical conditions, particularly cardiovascular, increased (Berg et al., 2010). Nevertheless, hemorrhage related to pregnancy remains a significant issue in the United States as seven causes of death, including hemorrhage, thrombotic pulmonary embolism, infection, hypertensive disorders of pregnancy, cardiomyopathy, cardiovascular conditions, and noncardiovascular medical conditions, each contributed 10% to 13% of maternal deaths (Berg et al., 2010). It is estimated that 70% to 92% of maternal deaths from hemorrhage are preventable (Berg et al., 2005). When considering all maternal deaths, the outcome of pregnancy was helpful in identifying specific risks. For example, no maternal deaths were reported as a result of a molar pregnancy, while 93% of deaths of women with ectopic pregnancy were hemorrhage related (Berg et al., 2010). When the outcome of pregnancy was live birth, maternal deaths were caused by a number of factors, including uterine rupture, placental abruption, placenta previa, placenta accreta, retained placental fragments, coagulopathies, and uterine atony (Berg et al., 2010). Significant causes of death in women whose pregnancy ends in stillbirth are hemorrhage from placental abruption and uterine rupture (Berg et al., 2010; Chang et al., 2003). Placental abruption and uterine rupture also are significant causes of fetal death (Silver, 2007).
As noted in The Joint Commission’s (2010) Sentinel Event Alert Preventing Maternal Death, the nurse must be alert to the symptoms of hemorrhage and shock and be prepared to act quickly to minimize blood loss and hasten maternal and fetal stabilization. Up to 15% of maternal cardiac output (750 mL to 1,000 mL/min) flows through the placental bed at term; unresolved bleeding can result in maternal exsanguination in 8 to 10 minutes (Rajan & Wing, 2010). In addition to the physiologic implications of bleeding during pregnancy,
the mother experiences emotional stress as she worries about the outcome for herself and her baby. Although maternal mortality decreased approximately 99% during the 20th century, hemorrhage remains a major cause of maternal death in all parts of the world. According to the most recent data, maternal mortality rates in the United States increased from 7.6 per 100,000 live births in 1996, 9.8 per 100,000 live births in 2000, and 12.1 per 100,000 live births in 2003 to approximately 15.4 per 100,000 live births in 2005 (Berg et al., 2010; Hoyert, 2007). The increase is significant as these rates had remained constant for the previous two decades (Clark et al., 2008) after many years of decreasing (e.g., 607.9 per 100,000 live births in 1915; 83.3 per 100,000 live births in 1950) (Hoyert, 2007). It is unclear whether the increase represents more women actually dying or improved coding and data collection methods (Berg et al., 2010).
the mother experiences emotional stress as she worries about the outcome for herself and her baby. Although maternal mortality decreased approximately 99% during the 20th century, hemorrhage remains a major cause of maternal death in all parts of the world. According to the most recent data, maternal mortality rates in the United States increased from 7.6 per 100,000 live births in 1996, 9.8 per 100,000 live births in 2000, and 12.1 per 100,000 live births in 2003 to approximately 15.4 per 100,000 live births in 2005 (Berg et al., 2010; Hoyert, 2007). The increase is significant as these rates had remained constant for the previous two decades (Clark et al., 2008) after many years of decreasing (e.g., 607.9 per 100,000 live births in 1915; 83.3 per 100,000 live births in 1950) (Hoyert, 2007). It is unclear whether the increase represents more women actually dying or improved coding and data collection methods (Berg et al., 2010).
Bleeding complicates approximately one in five pregnancies; the incidence and cause of bleeding vary by trimester (MacMullen, Dulski, & Meagher, 2005). Most bleeding occurring in the first trimester of pregnancy is related to spontaneous abortion and is generally not life threatening. Ectopic pregnancy is the leading cause of life-threatening hemorrhage during the first trimester, although the maternal mortality rate declined from 13.0% of all maternal deaths from 1970 to 1989 and to 6.0% from 1991 to 1999 (Creanga et al., 2011). Hemorrhage during the antepartum period usually results from disruption of the placental implantation site (involving a normally implanted placenta or placenta previa) (Hull & Resnik, 2009). Most serious obstetric hemorrhage occurs in the postpartum period as a result of uterine atony after placental separation (American College of Obstetricians and Gynecologists [ACOG], 2006; Driessen et al., 2011). Other causes of postpartum hemorrhage include retained placenta, uterine rupture, abnormal placental implantation, uterine inversion, genital tract trauma, and coagulopathy (ACOG, 2006; Oyelese & Ananth, 2010).
Symptomatic placenta previa is identified in approximately 0.3% to 0.5% of pregnancies (Harper, Obido, Macones, Crane, & Cahill, 2010; Oyelese & Smulian, 2006). Low implantation of the placenta is much more common during early pregnancy; however, most of these cases resolve or are not found to be clinically significant as pregnancy progresses (Harper et al., 2010; Oyelese & Smulian, 2006). Placentas may be classified as low lying during the second trimester by routine abdominal ultrasonography because it is difficult to determine which placentas cross the cervical os during ultrasonographic examination in early pregnancy. Transvaginal ultrasound remains a more accurate way to diagnose placenta previa and to measure the distance from the placental edge to the cervical os (Oyelese & Smulian, 2006; Vergani et al., 2009). The incidence of placenta previa is increasing, most likely secondary to the increasing cesarean birth rate (Hull & Resnik, 2009).
Placenta accreta is an uncommon abnormality of placental implantation where the placenta is abnormally adherent. Placenta accreta is one of the most serious complications of placenta previa. In addition to placenta previa, prior uterine surgery significantly increases the risk of placenta accreta (Comstock, 2011). With placenta previa, the risk of developing placenta accreta is 10% to 25% for women with a history of one prior cesarean birth and rises to more than 38% for women with two or more cesarean births or second-trimester pregnancy terminations (Comstock, 2011; Snegovskikh, Clebone, & Norwitz, 2011). The incidence of placenta accreta is rising secondary to an increase in the cesarean birth rate (Eller et al., 2011; Mahlmeister, 2010).
The incidence of abruptio placentae varies in the literature according to the population studied and diagnostic criteria. In the United States, the reported incidence of abruptio placentae is approximately 1% of all pregnancies (Hull & Resnik, 2009). Risk of recurrence in subsequent pregnancies has been reported to be as high as 5.5% to 16.6%. This rate is approximately 30 times higher than the rate for pregnant women without a history of prior abruptio placentae. The strongest risk factor for abruption is a history of placental abruption in a previous pregnancy (Hull & Resnik, 2009). The risk of recurrence for women with a history of two placental abruptions increases to approximately 25% (Hull & Resnik, 2009).
Vasa previa is a condition in which umbilical arteries and veins abnormally implanted throughout the amnion traverse the cervical os in front of the presenting part of the fetus. Vasa previa is a rare but life-threatening complication for the fetus at the time of rupture of membranes (Oyelese & Smulian, 2006; Robinson & Grobman, 2011). The reported incidence of vasa previa is approximately 1 in 2,500 births (Robinson & Grobman, 2011). Rupture of the vessels during spontaneous or artificial rupture of membranes usually leads to fetal exsanguination or severe neurologic fetal injury secondary to fetal hemorrhage before the cause of bleeding is recognized and before an emergent cesarean birth can be accomplished (Silver, 2007). Fetal death occurs in 60% to 75% of cases of ruptured vasa previa (Oyelese & Smulian, 2006).
Uterine rupture is another significant cause of maternal hemorrhage. The risk of uterine rupture for women attempting vaginal birth after cesarean birth (VBAC) is less than 1%; however, the consequences can be catastrophic for the mother and baby (ACOG, 2010; Spong & Queenan, 2011; Tillett, 2010). The risk of uterine rupture depends on the number, type, and location of the previous incisions (ACOG, 2010; Lang & Landon, 2010).
Although risk of uterine rupture has been reported as nearly five times greater for women with a history of two prior low transverse cesarean births when compared to women with one prior cesarean birth (ACOG, 2010), a large multicenter study did not find a difference in rupture rates in these two groups (Cahill & Macones, 2007; Landon et al., 2006; Lang & Landon, 2010). Women with a previous lowvertical uterine incision have a similar success rate for having a VBAC as those women with a previous low transverse incision (ACOG, 2010; Cahill & Macones, 2007). The risk of uterine rupture is increased for women with a T-shaped incision (ACOG, 2010).
Although risk of uterine rupture has been reported as nearly five times greater for women with a history of two prior low transverse cesarean births when compared to women with one prior cesarean birth (ACOG, 2010), a large multicenter study did not find a difference in rupture rates in these two groups (Cahill & Macones, 2007; Landon et al., 2006; Lang & Landon, 2010). Women with a previous lowvertical uterine incision have a similar success rate for having a VBAC as those women with a previous low transverse incision (ACOG, 2010; Cahill & Macones, 2007). The risk of uterine rupture is increased for women with a T-shaped incision (ACOG, 2010).
Waiting for spontaneous labor, thus avoiding pharmacologic cervical ripening agents and oxytocin, appears to significantly decrease the risk of uterine rupture for women attempting VBAC (ACOG, 2010; Landon et al., 2005; Lang & Landon, 2010). There are enough data to suggest that prostaglandins and high rates of oxytocin infusion increase the risk for rupture (ACOG, 2010; Lang & Landon, 2010). Uterine ruptures at the scar site and remote from the previous scar site have been reported with high doses of oxytocin (Lang & Landon, 2010). It has been theorized that prostaglandins induce local biochemical modifications that weaken the prior uterine scar, thus predisposing it to rupture (Lang & Landon, 2010). Due to the risk of uterine rupture with the use of misoprostol or any prostaglandin agent for cervical ripening or induction, ACOG (2010) does not recommend prostaglandins for women attempting VBAC. If labor needs to be induced in a patient with a previous scar for a clear and compelling clinical indication, the potential increased risk of uterine rupture with the use of prostaglandins should be discussed with the patient and documented in the medical record (ACOG, 2010).
The incidence of uterine inversion is approximately 1 case in 2,500 births, although the range varies among studies (You & Zahn, 2006). It is difficult to ascertain the true incidence because uterine inversion is not often reported in the literature. Improper management of the third stage of labor increases the likelihood of iatrogenic uterine inversion (Oyelese & Ananth, 2010).
Postpartum hemorrhage remains one of the leading causes of maternal death worldwide (Burke, 2010; Callaghan, Kuklina, & Berg, 2010; Oyelese & Ananth, 2010). Early or primary postpartum hemorrhage (within 24 hours after birth) is most frequently caused by uterine atony, retained placental fragments, lower genital tract lacerations, uterine rupture, uterine inversion, placenta accreta, and coagulopathies. Late or secondary postpartum hemorrhage (> 24 hours to 6 weeks after birth) is more likely to be caused by infection, placental site subinvolution, retained placental fragments, and coagulopathy (Rajan & Wing, 2010).
DEFINITIONS AND CLINICAL MANIFESTATIONS
The definitions, cause, pathophysiology, and clinical manifestations of the most frequently occurring causes of bleeding and bleeding disorders in pregnancy are described in the following sections. A diagnosis-specific summary of expected management is included. A more detailed summary of nursing interventions for bleeding during pregnancy concludes this section.
PLACENTA PREVIA
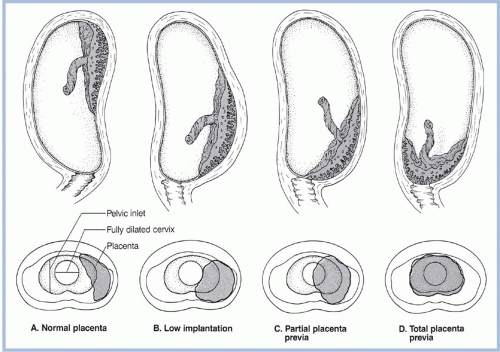
Placenta previa is the abnormal implantation of the placenta in the lower uterine segment. The reported incidence of placenta previa at birth varies widely. This variation results from differences in the time of diagnosis. Asymptomatic placenta previa is often diagnosed during routine ultrasound performed in the second trimester; most cases of placenta previa are detected before the third trimester (Oyelese & Smulian, 2006). These women are at increased risk for other obstetric complications such as placental abruption, intrauterine growth restriction (IUGR), and hemorrhage. It has been theorized that the placental tissue that surrounds the cervical os does not develop as well as the placental tissue that is in the myometrium (Hull & Resnik, 2009; Oyelese & Smulian, 2006). By the end of 40 weeks of pregnancy, the incidence of placenta previa is approximately 0.05% (Harper et al., 2010). The most significant risk factors include prior uterine surgery resulting in uterine scarring and history of a prior placenta previa (Hull & Resnik, 2009; Oyelese & Smulian, 2006). Late development and implantation of the ovum, more frequently occurring in older women, may also play a role in placenta previa. Display 6-1 lists the risk factors associated with placenta previa.
Previous placenta previa
Previous cesarean birth
Induced or spontaneous abortions involving suction curettage
Multiparity
Advanced maternal age (> 35 years)
Cigarette smoking
Multiple gestation
Fetal hydrops fetalis
Large placenta
Uterine anomalies
Fibroid tumors
Endometritis
African American or Asian ethnicity
Placental implantation has traditionally been classified as normal, low-lying, partial placenta previa, and total placenta previa (Fig. 6-1). Clark (1999) proposed a new classification system: placenta previa, in which the placenta covers the internal os in the third trimester, and marginal placenta previa, in which the placenta is within 2 to 3 cm of the internal os but does not cover the os. The rationale for this classification system is the ambiguity and lack of clinical utility of the term low-lying placenta. Until Clark’s (1999) classification, there had been no accepted definition of how close the placenta must be to mandate cesarean birth or double setup examination. It is now known that there is no increased risk of intrapartum hemorrhage if the distance from the lower margin of the placenta to the internal os is at least 2 to 3 cm (Vergani et al., 2009). As the ability to more accurately visualize placental location increases because of advancements in ultrasound technologies, this classification system has been adopted in clinical practice. The term placental migration (a misnomer) has been used to describe the apparent movement of the placenta away from the cervical os. The placenta does not move; it remains in place as the uterus expands away from the os.
Clinical Manifestations
Painless uterine bleeding during the second or third trimester characterizes placenta previa. The first significant bleeding episode may occur before 30 weeks’ gestation; some women never exhibit bleeding as a symptom until labor develops (Hull & Resnik, 2009). Rarely is the first bleeding episode life threatening or a cause of hypovolemic shock. The bright red bleeding may be intermittent or continuous. After the bleeding episode, women may demonstrate “spotting” of bright red or dark brown blood on the peripad.
Diagnosis
The standard for the diagnosis of placenta previa is an ultrasound examination. It may be a transabdominal, transvaginal, or translabial ultrasound. Transvaginal ultrasound provides precise information regarding the placement of the placenta in relation to the cervical os (Hull & Resnik, 2009; Oyelese & Smulian, 2006). If ultrasound reveals a normally implanted placenta, a speculum examination is performed to exclude local causes of bleeding (e.g., cervicitis, polyps, carcinoma of the cervix) and a coagulation profile is obtained
to exclude other causes of bleeding. Diagnosis of placenta previa increased dramatically with the advent of transabdominal ultrasound; the rate has decreased with the use of transvaginal or translabial ultrasound (Oyelese & Smulian, 2006). Placenta previa is most often diagnosed before the onset of bleeding when an ultrasound examination is performed for other indications.
to exclude other causes of bleeding. Diagnosis of placenta previa increased dramatically with the advent of transabdominal ultrasound; the rate has decreased with the use of transvaginal or translabial ultrasound (Oyelese & Smulian, 2006). Placenta previa is most often diagnosed before the onset of bleeding when an ultrasound examination is performed for other indications.
Management
Conservative management is usually possible when the fetus is not mature and maternal status is stable. When survival is likely and fetal lung maturity is achieved, birth can be accomplished. Most births are by cesarean section, although vaginal birth may be achieved if the placental edge does not completely cover the cervical os. This type of vaginal birth occurs in the operating room with personnel and equipment available for a cesarean birth if needed (i.e., a double setup).
Patients are frequently hospitalized with the initial bleeding episode. Those with recurrent bleeding episodes, recurrent uterine activity associated with bleeding, or evidence of fetal or maternal compromise usually remain hospitalized until the birth. Some women will have a life-threatening bleeding event. For women who are unstable, blood can be cross-matched at all times and intravenous (IV) access maintained. For stable patients with occasional spotting, a saline lock may be used to maintain an IV access site. In the event of sudden-onset hemorrhage, a second IV line should be initiated with a large bore catheter because it is very difficult to obtain IV access when the woman is in shock. Women who experience an initial bleeding episode that resolves, are hemodynamically stable, demonstrate fetal well-being, and have emergency services readily available to them are candidates for expectant management as outpatients (Hull & Resnik, 2009; Oyelese & Smulian, 2006).
ABRUPTIO PLACENTAE
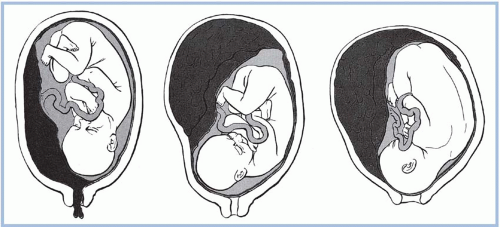
Abruptio placentae, or premature separation of the placenta, is the detachment of part or all of the placenta from its implantation site, typically occurring after the 20th week of pregnancy (Fig. 6-2). Premature separation of the placenta is a serious event and accounts for about 15% of all neonatal deaths (Witlin, Saade, Mattar, & Sibai, 1999). More than 50% of these deaths are the result of preterm birth. Other causes of fetal death include hypoxia and asphyxia. Risk factors associated with abruptio placentae are listed in Display 6-2. Despite these reported risk factors, the exact cause of abruptio placentae is unknown. There may be some type of disease or damage to the blood vessels; this may be of long duration. The risk of recurrence in subsequent pregnancies has been reported as high as 5% to 16% (Hull & Resnik, 2009). Women with two previous placental abruptions have a risk of recurrence of 25% (Hull & Resnik, 2009). Women with severe preeclampsia and eclampsia are at high risk for abruptio placentae. This high-risk status includes women with mild pregnancy-induced or chronic hypertension. A placental abruption significant enough to cause fetal death is less common (1 in 420 births), but as use of cocaine has increased, fetal death associated with abruptio placentae has risen in selected populations (Hull & Resnik, 2009).
Quantitative proteinuria and the degree of blood pressure elevation are not predictive of an abruption (Witlin et al., 1999). Investigators concluded that the greatest morbidity occurred in preeclamptic women with preterm gestations not receiving prenatal care (Witlin et al., 1999). Thrombophilias, such as factor V Leiden or the antiphospholipid antibody syndrome, were thought to be associated with an increased risk of abruption (Sibai, 2005); however, studies have shown that there is no increased risk of abruption with factor V Leiden mutation (Hull & Resnik, 2009).
Quantitative proteinuria and the degree of blood pressure elevation are not predictive of an abruption (Witlin et al., 1999). Investigators concluded that the greatest morbidity occurred in preeclamptic women with preterm gestations not receiving prenatal care (Witlin et al., 1999). Thrombophilias, such as factor V Leiden or the antiphospholipid antibody syndrome, were thought to be associated with an increased risk of abruption (Sibai, 2005); however, studies have shown that there is no increased risk of abruption with factor V Leiden mutation (Hull & Resnik, 2009).
Partial abruption of current pregnancy
Prior abruptio placentae
Rapid decompression of the uterus, such as birth of the first of multiple fetuses and amniotic reduction therapy in polyhydramnios
Hypertension
Preterm premature rupture of membranes <34 weeks’ gestation
Prior cesarean birth
Blunt abdominal trauma
Thrombophilias
Multiparity
Cocaine use
Cigarette smoking
Extremely short length of the umbilical cord
Uterine anomalies
Uterine fibroids at the placental implantation site
Use of intrauterine pressure catheters during labor
Clinical Manifestations
Abruptio placentae is suspected in the woman presenting with sudden-onset, intense, often localized uterine pain or tenderness with or without vaginal bleeding. Another common presentation is preterm contractions with vaginal bleeding or with an occult abruption in the absence of abdominal pain. The woman may also present with painless vaginal bleeding, although this is uncommon. In mild cases, the pain from abruption may be difficult to distinguish from the pain of labor contractions. In many cases, pain is localized to the area of the abruption. When placental implantation is posterior, lower back pain may be more prominent than uterine tenderness. Occasionally, nausea and vomiting may occur. Vaginal bleeding from an abruptio placentae is not usually proportional to the degree of placental detachment because blood may become trapped behind the placenta. If the abruption is located centrally, no vaginal bleeding is visualized initially. Approximately 10% of women present with concealed hemorrhage (Hull & Resnik, 2009). Marginal separations and large abruptions are associated with bright red bleeding and are almost always accompanied by contractions that are usually of low amplitude and high frequency (Hull & Resnik, 2009; Oyelese & Ananth, 2010; Oyelese & Smulian, 2006).
Contractions may be difficult to record if there is an increase in uterine resting tone and for women at earlier gestations. Palpation for uterine contractions or hypertonus is necessary. The contraction pattern of women with an evolving placental abruption often will show frequent contractions of short duration. Fetal assessment by electronic fetal monitoring (EFM) should be accomplished before obtaining a full uterine ultrasound because most placental abruptions cannot be accurately identified with ultrasonography. More than 50% of placental abruptions are not visible on ultrasound (Hull & Resnik, 2009; Oyelese & Ananth, 2010).
The fetal response to abruptio placentae depends on the volume of blood loss and the extent of uteroplacental insufficiency. Anticipatory nursing care includes being alert to the possibility of an abruption in the presence of any or all of the following: fetal tachycardia; bradycardia; loss of variability; presence of late decelerations; decreasing baseline (especially from tachycardia to a normal or near normal baseline with minimal or absent variability); a sinusoidal fetal heart rate (FHR) pattern; low-amplitude, high-frequency contractions; uterine hypertonus; and abdominal pain.
The Kleihauer-Betke (KB) test may be performed on the mother’s serum or vaginal blood to test for the presence of fetal red blood cells (RBCs). Fetal-to-maternal transfer of blood is documented by the presence of fetal cells in maternal serum (Silver, 2007). Depending on fetal age and size, the number of fetal RBCs present in maternal blood can be calculated to estimate the fetal blood loss. Formulas for this calculation can be found in the obstetrical literature (Cunningham et al., 2010). Cesarean birth is not always indicated. The decision to proceed with cesarean birth is usually based on fetal status. In the setting of a normal FHR tracing, expectant management may be appropriate for those women who are preterm and not in labor, providing the abruption is small and the mother and fetus are stable. Labor or cesarean birth, whichever mode presents the fewest risks to the woman and/or fetus, is indicated for significant bleeding or coagulopathy. The woman should be stabilized hemodynamically and hematologically before proceeding with labor initiation or cesarean birth. Some women with an abruption may demonstrate very rapid labor progress (Hull & Resnik, 2009). Chronic abruptio placentae may develop with the woman experiencing episodic bleeding, subjecting the fetus to prolonged stress and increased risk of IUGR (Hull & Resnik, 2009; Oyelese & Ananth, 2010). Risk of developing disseminated intravascular
coagulation (DIC) exists during placental abruption because of release of thromboplastin from the site into the maternal bloodstream.
coagulation (DIC) exists during placental abruption because of release of thromboplastin from the site into the maternal bloodstream.
Diagnosis
The diagnosis of abruptio placentae is based on the woman’s history, physical examination, and laboratory studies. Examination of the placenta at birth or by a pathologist confirms the diagnosis. Ultrasonography is used to exclude placenta previa; however, it is not diagnostic for abruption (Hull & Resnik, 2009). Abruptions are classified as partial, marginal (i.e., only the margin of the placenta is involved), or total (i.e., complete).
Management
Treatment depends on maternal and fetal status. In the presence of fetal compromise, severe hemorrhage, coagulopathy, poor labor progress, or increasing uterine resting tone, an emergent cesarean birth is performed once efforts to stabilize the woman have been initiated. In an older but reliable study, 22% of all perinatal deaths from abruption occurred after the patient was hospitalized, with 30% occurring in the first 2 hours (Knab, 1978). If the mother is hemodynamically stable, the fetus is alive with a normal FHR tracing (or an indeterminate FHR tracing with imminent birth), or if the fetus is dead, a vaginal birth may be attempted. If the mother is hemodynamically unstable, attempts are first directed at maternal stabilization.
IV access is established; if possible, two lines are placed. Blood replacement products and lactated Ringer’s solution are infused in quantities necessary to maintain urine output of 30 to 60 mL/hr and a hematocrit of approximately 30%. Some experts suggest 1 Unit of blood replacement for every 4 L of IV fluid or 3 mL of crystalloid solution for every milliliter of blood loss (Clark, 2004). Blood loss is almost always underestimated (Oyelese & Smulian, 2006). Fluid resuscitation is aggressive in the presence of hemorrhage. With rapid volume IV infusions, the nurse anticipates the possibility of pulmonary edema due to lower colloid osmotic pressure in pregnancy. DIC may develop, placing the mother at significant risk for maternal morbidity and mortality.
ABNORMAL PLACENTAL IMPLANTATION
Abnormal adherence of the placenta occurs for unknown reasons but is thought to be the result of zygote implantation in an area of defective decidua basalis (Comstock, 2011). The risk has greatly increased in the last 10 years with the rapidly rising rate of cesarean births (Eller et al., 2011; You & Zahn, 2006). The risk of placenta accreta increases with the number of previous cesarean births; the odds ratio increases from 1.3 for a second cesarean birth to 29.8 for the sixth or greater cesarean births (ACOG, 2006; Comstock, 2011; Wright et al., 2011). When pregnancy is complicated by placenta previa, the risk of accreta is much greater, increasing to 67% for women with four or more cesarean births presenting with anterior or central placenta previa (Lyndon et al., 2010; Wright et al., 2011). Patients with one prior cesarean birth who present with anterior or central placenta previa in the subsequent pregnancy have a 24% risk of placenta accreta (ACOG, 2002). Placenta accreta and uterine atony are the two most common causes of postpartum hysterectomy (ACOG, 2006; Eller et al., 2011). Other risk factors include advanced maternal age, smoking, and a short interconceptual period.
Clinical Manifestations
Placenta accreta occurs when there is a lack of decidua basalis, so that the placenta is implanted directly into the myometrium. Complete accreta occurs when the entire placenta is adherent; partial accreta occurs with one or more cotyledons adherent; and focal accreta occurs with one piece of a cotyledon adherent. Placenta increta is the abnormal invasion of the trophoblastic cells into the uterine myometrium. Placenta percreta occurs when the trophoblast cells penetrate the uterine musculature and the placenta develops on organs in the vicinity of the percreta. Placenta percreta can adhere to the bladder and other pelvic organs and vessels. Placenta percreta accounts for only 5% to 7% of cases of abnormal adherence. Placenta increta occurs in 15% to 18% of cases, and placenta accreta is the most common form, accounting for 75% to 80% of cases (Comstock, 2011




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access