Arteriovenous Malformations and Other Cerebrovascular Anomalies
Deidre A. Buckley
Joanne V. Hickey
Cerebrovascular malformations are believed to be developmental vascular anomalies that result from failure of the embryonic vascular network to develop properly.1 The incidence of cerebrovascular malformations is unclear because many lesions are asymptomatic and are found only incidentally at autopsy. They are relatively uncommon lesions that can cause a variety of symptoms. Some lesions present with serious symptoms such as hemorrhage, seizure, or headaches, whereas other lesions are quite benign with no symptoms at all. The presentation, location, and natural history of these lesions will determine whether management will be surgical, endovascular, radiosurgical, or observation.
The authors would like to acknowledge Michael Phillips, BA for his contributions of vignettes, and also his review and suggestions related to content for the chapter.
CLASSIFICATIONS OF VASCULAR MALFORMATIONS
Cerebrovascular malformations are classified into five major categories: capillary telangiectases, venous malformations (VMs), arteriovenous malformations (AVMs), dural arteriovenous fistulas (DAVFs), and cavernous malformations (CMs).2 The term malformation is preferable to the previously used angioma. Angioma has a connotation of neoplasia, a connotation that is inaccurate with these lesions. The incidence of vascular malformations varies from 0.1% to 4% in various autopsy studies. In a large autopsy series, the detection of AVMs was 1.4% (46 AVMs among 3,200 brain tumor cases). Vascular malformations are about one seventh as common as intracranial saccular aneurysms. AVMs are the most common type of vascular lesion, followed by CMs and VMs. With improvement in cross-sectional imaging in both computed tomography (CT) and magnetic resonance imaging (MRI), higher detection rates are being reported and in patients with increasing age.2 Because some of these lesions do not have direct arterial vessel input, they may not be visible on angiography.1, 3, 4
Telangiectases
Telangiectases are small (0.3 to 1 cm) capillary lesions that are composed of clusters of vessels that look something like dilated capillaries separated by normal-appearing parenchyma. These lesions have little clinical significance because they rarely cause any symptoms; they are seldom apparent on radiologic examination such as CT scan, but are visible on contrast-enhanced MRI, which is the preferred modality. Capillary telangiectasias are typically angiographically occult.5 Telangiectases are an incidental finding at autopsy and can be found anywhere in the brain and spinal cord but are most commonly located in the posterior fossa and have an overall frequency of 0.1%.2 A different analysis performed at post-mortem indicated a prevalence as high as 0.4%.6
Cavernous Malformations
CMs, also called cavernous hemangiomas, cavernous angiomas, and cavernomas, are congenital nodular lesions. They resemble a mulberry or “popcorn-like” appearing lesion in the brain, spinal cord, or nerve roots and are composed of sinusoidal-type vessels that are not separated by normal-appearing parenchyma (neural tissue). Microscopic examination often reveals small hemorrhages with numerous, hemosiderin-laden macrophages and gliotic tissue in the adjacent parenchyma. Elastic fibers are absent in the walls of these vascular caverns. Thrombosis may be present in some of the dilated venules.2 Calcification within the lesion is common.
CMs are well-defined, purple lesions that may grow to an appreciable size and may be mistaken for a brain tumor on CT scan. Other CMs are found incidentally at autopsy. The overall frequency of CMs was approximately 0.4% in a large autopsy study. A retrospective review found a detection rate of 0.4% to 0.9% on MRI studies. CMs are rare in children and account for about 10% of all symptomatic vascular malformations.2 The peak occurrence is in the third and fifth decades of life.7 CMs occur in two forms, either sporadic, which is characterized by one lesion, or familial, which is characterized by multiple lesions with an autosomal dominant mode of inheritance.8 Genetic linkage studies revealed a locus for CM on chromosome 7q. Two additional loci have also been identified mapping to chromosomes 7p and 3q. The CM gene was successfully identified as KRIT1.7
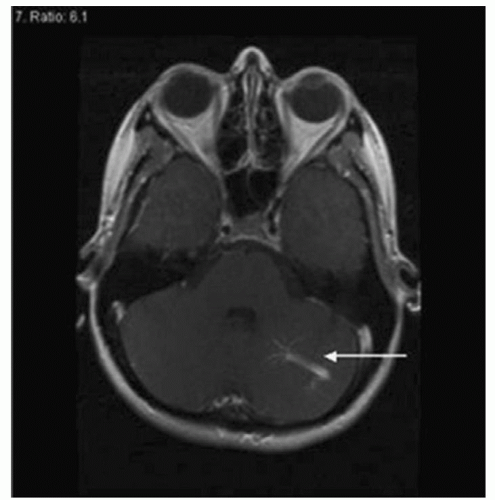
These low-flow lesions are not apparent on angiography, but may be visualized on CT scan and MRI (Fig. 25-1) as a combination of high and low T1 and T2 signals with surrounding hemosiderin. Most often, CMs present on MRI with no symptoms. Some CMs may cause seizures and intracerebral hemorrhage. Symptom presentation depends on location and lesion size. A CM of any size can present with hemorrhage. These hemorrhages tend to occur at lower pressures and with less severity
than those bleeds associated with AVMs, although serious hemorrhages that are life-threatening or those with serious consequences can occur with CMs of the cerebellum or brainstem. The frequency of hemorrhage among those who present with either incidental diagnosis or seizures is approximately 0.4% to 2% per year. In those who present with hemorrhage, the annual recurrence rate is higher, with a rate of about 4% to 5% in the next year. Location also plays an important role in hemorrhage risk. Patients with brainstem, thalamic, cerebellar, or basal ganglia lesions have an initial annual hemorrhage risk of 4.1% compared to 0.4% among those with superficial lesions.2
than those bleeds associated with AVMs, although serious hemorrhages that are life-threatening or those with serious consequences can occur with CMs of the cerebellum or brainstem. The frequency of hemorrhage among those who present with either incidental diagnosis or seizures is approximately 0.4% to 2% per year. In those who present with hemorrhage, the annual recurrence rate is higher, with a rate of about 4% to 5% in the next year. Location also plays an important role in hemorrhage risk. Patients with brainstem, thalamic, cerebellar, or basal ganglia lesions have an initial annual hemorrhage risk of 4.1% compared to 0.4% among those with superficial lesions.2
Treatment is considered in patients who present with multiple episodes of hemorrhage that are either clinically or radiographically significant or in patients suffering from uncontrolled seizures. The optimal treatment is stereotactic surgical assistance for the supratentorial lesions. Many surgeons choose to observe lesions in critical areas such as the brainstem, basal ganglia, central sulcus, and spinal cord. They may be pushed to treatment with repetitive hemorrhages. The hemorrhage can create a clot cavity, thus providing a route for surgical access and creating a plane for dissection of the lesion.
It is unclear whether radiosurgery has any effect on CMs compared with the natural history. Some groups have noted that radiosurgery may have a greater risk of morbidity compared with AVM radiosurgery. Surgical resection can be performed safely in some patients with deep CMs. Only a select group of patients are considered candidates for radiosurgery. This group would include patients with repetitive hemorrhages where surgery would have prohibitive risks.2
Venous Malformations
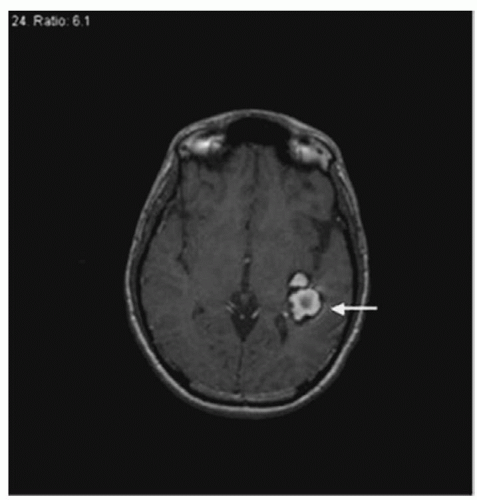
VMs, also referred to as venous anomalies, are composed of anomalous veins, separated by normal parenchyma, which drain into a dilated venous trunk. A VM is important to brain tissue because it provides normal venous drainage. VMs are the most common vascular anomaly of the brain and are detected in up to 2.6% of all performed autopsies.2 These lesions have no recognizable direct arterial input. Calcification within the lesion is rare. The predominant location of VMs is in the cerebrum, although some are found in the cerebellum (3:1 ratio). Clinically, the patient may present with seizures. VMs may be evident on CT scan, MRI (Fig. 25-2), or angiography. Those found in the cerebrum rarely cause hemorrhage and are treated conservatively. At times, venous anomalies may be adjacent to CMs. If the CM is symptomatic such that the patient presents with hemorrhage, the CM may need to be removed. If there is an adjacent venous anomaly, special care must be taken during surgical resection of the CM, as damage to the venous anomaly may lead to stroke. Rarely, vascular malformations may be associated with seizures, motor symptoms, trigeminal neuralgia, or motor deficits. These lesions are simply an anomalous pattern of functionally normal venous drainage. Any attempt to resect or treat may result in occlusion, which frequently causes a venous infarction. Therefore, resection of VMs is not recommended, and conservative management is the goal.1
Dural Arteriovenous Malformations (Fistulas)
Dural AVMs, also called DAVFs, are almost always acquired lesions, rather than developmental. Various causes of acquired dural AVMs have been proposed such as trauma, surgery, and infection. There has also been an association with certain diseases such as Osler-Weber-Rendu as well as an association with pregnancy.9
Dural AVMs comprise 10% to 15% of all intracranial AVMs. They are defined by the following criteria: (1) the nidus of the arteriovenous (AV) shunting is within the cranial dura mater; (2) arterial supply arises exclusively from the extracranial circulation or
from the meningeal branches of the intracranial branches; and (3) venous drainage is either directly into the dural venous sinus or into nearby leptomeningeal veins.10 Dural AVMs can occur in either the cranial or spinal areas of the central nervous system. There is a higher incidence in women between 30 and 50 years of age.9
from the meningeal branches of the intracranial branches; and (3) venous drainage is either directly into the dural venous sinus or into nearby leptomeningeal veins.10 Dural AVMs can occur in either the cranial or spinal areas of the central nervous system. There is a higher incidence in women between 30 and 50 years of age.9
Venous outflow obstruction, at times associated with sinus thrombosis, can precede development of dural AVMs. The clinical presentation of dural AVMs is primarily related to the pattern and location of venous drainage, rather than to the characteristics of the nidus of the malformation or the arterial feeders.11 Clinical symptoms are manifested as generalized nervous system signs (e.g., papilledema, headache, visual disturbances, or hydrocephalus due to malabsorption of the cerebrospinal fluid) and focal signs (e.g., seizure, transient ischemic attack, intraparenchymal hemorrhage, and fixed cortical, brainstem, and cerebellar deficits). The general symptoms are related to venous hypertension in the dural sinuses and the focal symptoms indicate direct or indirect retrograde cortical venous drainage. Leptomeningeal venous drainage is the most important risk factor for focal neurological deficits and hemorrhage.10 Symptoms of lateral and sigmoid sinus dural AVMs include a pulse-synchronous tinnitus or bruit (70% of cases), papilledema, and headaches, while anterior cranial fossa and tentorial dural AVMs often present with intradural bleeding in 84% and 71% of cases, respectively.10
The natural history of dural AVMs is highly variable, ranging from spontaneous regression of the lesion, to stable persistence with minor clinical manifestations, to gradual progression to major neurological disability, to acute intradural hemorrhage. Pulsatile tinnitus often occurs with lesions of the transverse or sigmoid sinus.12 Cortical-based lesions may cause seizures or progressive deficits. With cavernous sinus lesions exophthalmos, diplopia or impaired vision may occur. A superior sagittal sinus lesion may cause papilledema, visual loss, and benign intracranial hypertension (formerly called pseudotumor cerebri).
A brain CT scan usually does not detect dural AVMs, but may detect blood, abnormal density in the white matter (due to venous congestion), mass effect, hydrocephalus, and dilation of major venous sinuses while failing to reveal the lesion.12 An MRI is more sensitive in detecting abnormal enhancement near the bony cranial vault, dilated pial veins, or thrombosed venous sinuses. A fourvessel angiogram with selective external carotid artery (ECA) injection is still the “gold standard” for treatment planning.2 Computed tomography angiography (CTA) is a newer diagnostic tool that is helpful in localizing a dural AVM.
There have been a number of classification systems developed to help describe and explain the angiographic anatomy of dural AVMs. The purpose of the classification is to determine risk of hemorrhage so that appropriate treatment can be selected. A physician uses the classification systems to define venous drainage of the dural AVM to assist in the treatment plan. Some of the most commonly used classification systems are the Djindjian system, the Borden classification system, and the Cognard system.
In 1978, Djindjian describes the venous drainage via a three-tiered system.
Type 1 lesions drain into the ispsilateral sinus.
Type 2 lesions drain into the contralateral sinus.
Type 3 lesions drain directly into a cortical vein.
Later, there was a fourth group identified as type 4 lesions in which the dural AVM drains via the cortical vein with an associated venous pouch. Type 1 lesions are typically benign with low risk for hemorrhage and usually present with headache or bruit. Those patients with type 2 lesions are at high risk for hemorrhage, and patients with types 3 and 4 lesions are at highest risk for hemorrhage.9
The Borden classification system is user friendly with three types described.
Type 1: dural AVM drains into the dural sinus with normal anterograde flow and with a benign presentation.
Type 2: dural AVM drains into the dural sinus with anterograde flow and retrograde drainage into the cortical veins. Patients with a type 2 lesion usually present with hemorrhage.
Type 3: dural AVM has direct retrograde flow from the fistula into the cortical veins. Drainage is retrograde through a vein that normally drains into a sinus.13
The Cognard classification system is a five-tiered system that details the direction of flow, whether retrograde, anterograde or normal and notes the presence or absence of cortical venous drainage. The presence of direct cortical venous flow is a strong predictor of hemorrhage. The classification is as follows.
Type I: venous drainage into sinus with normal anterograde flow.
Type IIA: venous drainage into sinus with retrograde flow into sinus.
Type IIB: venous drainage into sinus with retrograde flow into cortical veins.
Type II A+B: venous drainage into sinus with retrograde flow into both sinus and cortical veins.
Type III: venous drainage into cortical veins.
Type IV: venous drainage directly into cortical veins with venous dilatation (ectasia).
These systems are very useful in helping the clinician determine the importance of the clinical anatomy of the lesions in association with clinical manifestations in order to proceed to appropriate treatment. For example, studies revealed that the presence of direct cortical venous drainage was a strong predictor of hemorrhage.14
Management of dural AVMs often requires a multiple modality approach. Some DAVFs can be managed conservatively if they are benign in nature. It is believed there may be regression of the fistula caused by thrombosis of the sinus or fistula. This is most commonly seen in the cavernous sinus fistulas. In other situations, urgent treatment may be required if the patient presents with acute severe symptoms such as hemorrhage (intraparenchymal or subarachnoid) or progressive visual loss. Those patients with proptosis, intractable pulsatile tinnitus, or chemosis may be considered for curative, or at least palliative, treatment.2 Treatment of DAVFs has evolved over the last 4 decades.
Transarterial embolization, transvenous embolization, and surgical resection of the lesion may be used alone or in combination depending on the anatomy of the lesion. A patient may be transferred to a tertiary care center so that they may be treated by clinicians capable of multi-modality treatments. Stereotactic radiosurgery may also be used, although currently reported clinical trials are not clear of its role in treatment.15
Treatment modality is based on careful decision making by the clinician, with proper knowledge of anatomical drainage patterns, aggressiveness of symptoms, and available treatment resources and expertise. Today, endovascular techniques are used for the majority of DAVFs of cavernous and inferior petrosal and marginal sinus. Surgery is often used with DAVFs located in the ethmoidal region. Each case requires careful consideration of the patient’s symptoms
and angiographic anatomical presentation, as well as the skill of the clinician and the technology available at the institution.
and angiographic anatomical presentation, as well as the skill of the clinician and the technology available at the institution.
Arteriovenous Malformations
An AVM is composed of a tightly tangled collection of abnormalappearing, dilated blood vessels that directly shunt arterial blood into the venous system without the usual connecting capillary network. Blood vessels of the AVM are thin walled and tortuous and lack the normal characteristics of veins or arteries. The three morphologic components of an AVM are the nidus, the feeding arteries, and the draining veins. (The literature often refers to the nidus when discussing AVMs; a nidus is defined as the focus of the AVM, that is, the tangle of abnormal vessels). The vessels of the AVM vary greatly in diameter, but the veins are generally larger than the arteries. The arterial vessels, also called feeder arteries, supply the AVM. Dilated veins without the usual intervening capillary network drain the lesion. As a result of the absence of the capillary network, blood flow is accelerated and the pressure is elevated within the fragile vessels of the AVM. These conditions predispose the lesion to hemorrhage.
AVMs may be small and focal, or they may be large, involving an entire hemisphere. Some are conical, with the apex pointing inward and the base positioned on the surface of the cerebral cortex. In rare instances, the lesion is so deep that the ventricles and choroid plexus are involved, thus predisposing the person to intraventricular hemorrhage. The parenchyma between the vessels of the AVM is usually abnormal, with nonfunctional gliotic tissue (proliferation of neuroglia [i.e., supporting tissue] in the brain). Frequently, there is evidence of old hemorrhage and hemosiderin deposits (ironcontaining glycoprotein pigment found in tissue, excessive amounts of which occur in pathologic conditions). Patients may have evidence of a radiographic hemorrhage without clinical signs or symptoms of hemorrhage.
The AVM is the most common cerebrovascular lesion that causes symptoms and is, therefore, clinically significant. Over the last decade, there have been significant developments in the treatment of intracranial AVMs. There has been an evolution in microsurgical, endovascular, and radiosurgical techniques to treat these lesions. As the management options have evolved, combined modality treatment protocols have developed. Both children and adults may be affected by AVMs. However, AVMs in adults constitute the focus of most of this chapter.
ARTERIOVENOUS MALFORMATIONS IN THE ADULT: AN OVERVIEW
Several important points can be made to describe AVMs, they account for 8.6% of subarachnoid hemorrhages and 1% of strokes are attributable to ruptured AVMs. The Harvard Cooperative Stroke Registry conducted a 3-year study documenting 9 AVM cases among 494 cases of stroke from all causes of stroke in a population of 1,00,000. This yields an incidence of about 3 per 1,00,000 annually. The ratio of AVMs to intracranial aneurysms is 1:10.3,16 Approximately 90% of AVMs are located in the supratentorial area and involve the cerebral hemispheres; only 10% of this group are located in the deep subcortical areas (e.g., basal ganglia, thalamus, corpus callosum). The location of 10% of AVMs is within the cerebellum and brainstem. Of those patients with AVMs, 80% develop symptoms between the ages of 20 and 40 years; the remaining 20% develop symptoms before the age of 20 years.
Pathophysiology and Pathologic Characteristics of Parenchymal Arteriovenous Malformations
There are two pathophysiologic characteristics related to AVMs. The first is the effect of shunting of blood from the arterial to the venous system without the intervening capillary network. Normally, the capillary network provides capillary resistance to blood flow, thus decreasing the intravascular pressure. However, when an AVM is present, blood is shunted from the high-resistance normal vascular bed to the low-resistance vessels within the AVM, thus exposing the draining venous channels to elevated intravascular pressure. These dynamics predispose the vessels to rupture and hemorrhage.
The second characteristic is the effect of impaired perfusion of the cerebral tissue adjacent to the AVM. Elevated intravascular and venous pressure impairs cerebral perfusion pressure. When the AVM is large and has a high flow, the diversion of blood to the AVM may cause ischemia to the adjacent normal tissue. Clinically, this is evidenced by slowly progressive neurological deficits. The diversion of blood to the AVM is called the vascular steal phenomenon.
Parenchymal AVMs result from abnormalities in the vasculature during fetal development from fetal age of 3 to 12 weeks. Failure of normal involution of embryonic vasculature networks is thought to occur as AVMs develop. Some AVMs may enlarge by recruitment of new blood vessels during childhood and early adulthood.1
AVMs are circumscribed vascular lesions that displace, rather than encompass, normally functioning brain tissue. They are separated from normal tissue by abnormal and nonfunctional gliotic parenchyma. Evidence of microscopic or gross hemorrhage is very common. This is true even in patients who have no clinical manifested hemorrhage. Partial thrombosis of an AVM is not uncommon and can occur spontaneously. This may explain why some AVMs appear smaller on repeat angiography than on a previous study. This may also explain why some AVMs that are classified as “occult” are not seen on angiography.
Clinical Presentation
The major clinical presentation of AVMs is hemorrhage, seizures, headache, and progressive neurological deficits. The following section provides a brief discussion of each symptom.
Hemorrhage. Hemorrhage is the most common initial manifestation of AVMs. More than 50% of AVMs present with intracranial hemorrhage.17 When an AVM ruptures, it usually causes intraparenchymal hemorrhage.18, 19 If the AVM is superficial, a subarachnoid hemorrhage can occur. In the rare instance of an AVM that involves the choroid plexus, rupture can result in intraventricular hemorrhage. The available natural history studies demonstrate an overall risk of initial hemorrhage of approximately 2% to 3% per year.20 Mortality associated with initial AVM rupture is reported to be between 6% and 30% with an average of about 15%. Serious morbidity is reported to be between 15% and 80% with an average of about 30%. The combined morbidity and mortality rates from an AVM bleed may be as high as 15% to 80%.21, 22, 23, 24 The chance of rebleeding during the first year is approximately 6%.25 After the first year, the incidence of recurrent hemorrhages decreases to 4% per year. This is the same as the rate of hemorrhage from AVMs that have never bled. In addition to annual risk of
hemorrhage, the lifetime risk must be considered. With the assumption of a 2% to 4% annual hemorrhage risk, the life-time risk of a bleed among people with previously unruptured AVMs is calculated by the following formula: lifetime risk (percentage) = 105 minus the patient’s age in years.26 The hemorrhage associated with rupture of an AVM is not as devastating as that associated with a ruptured cerebral aneurysm. In addition, the vasospasm and acute rebleeding (20% to 40% aneurysms rebleed within 14 days) associated with aneurysms are not characteristic of AVMs.4 Vasospasm does not occur within the AVM, because, there is no blood in the basilar subarachnoid cistern around the major intracranial arteries as is commonly found with aneurysmal rupture.
hemorrhage, the lifetime risk must be considered. With the assumption of a 2% to 4% annual hemorrhage risk, the life-time risk of a bleed among people with previously unruptured AVMs is calculated by the following formula: lifetime risk (percentage) = 105 minus the patient’s age in years.26 The hemorrhage associated with rupture of an AVM is not as devastating as that associated with a ruptured cerebral aneurysm. In addition, the vasospasm and acute rebleeding (20% to 40% aneurysms rebleed within 14 days) associated with aneurysms are not characteristic of AVMs.4 Vasospasm does not occur within the AVM, because, there is no blood in the basilar subarachnoid cistern around the major intracranial arteries as is commonly found with aneurysmal rupture.
Seizures. Seizures are the second most common manifestation of AVMs.18, 20, 25 Seizures occur in approximately 20% to 25% of patients.20, 27, 28 It has been suggested that approximately 70% of patients with AVMs have seizures at some time.19 Of those patients who have seizures, about 50% experience the focal type. The natural history of a patient with an AVM presenting with seizures is still not certain.
The etiology of seizures in patients with AVMs is also not clear, but two explanations have been suggested. First, cortical injury from hemorrhage into areas adjacent to the AVM may cause foci for seizure activity. Second, chronic ischemia from the vascular steal phenomenon may also cause abnormal cells that may become the foci for seizure activity.16
In a patient who presents with seizure, there is a 25% chance of hemorrhage within 15 years, or 4% per year.29 Medical therapy is generally successful in the control of seizure activity. The prognosis for patients who have surgery or embolization in combination with surgery to resect the AVM decreased the frequency of seizures in most patients.30 Seizure activity usually improves after radiosurgery.31
Headache. Other presentations of AVMs include headaches seen in 15% of affected patients.32 Recurrent headache that does not respond to usual drug therapy may be the only symptom of an AVM. Some patients experience migraine-like headaches. In some circumstances, headaches are clearly related to an underlying AVM, but in others, there is a much less well-defined relationship. A headache work-up with an MRI scan may identify the presence of an AVM incidentally. Often after the removal of the AVM, the patient’s headaches resolve.33
Progressive Neurological Deficits. The development of progressive neurological deficits is primarily attributed to cerebral ischemia resulting from vascular steal. The signs and symptoms will depend on the specific area of cerebral tissue deprived of adequate blood supply. Progressive deficits may also be related to repeated small hemorrhages that have not been clinically apparent.
Venous hypertension from arterialization of the venous system may account for neurological deterioration. Hydrocephalus is another mechanism that plays a role in progressive neurological deficits caused by ventricular compression from dilated veins.
Neuropsychiatric Manifestations
AVMs may cause neuropsychiatric symptoms. Approximately 10% of patients with an AVM manifest some sort of neuropsychiatric behavior. The vascular steal syndrome is thought to be responsible for such symptoms.16
Diagnosis
Diagnostic Procedures
CT Scan (CT). The CT scan is one of the initial screening tools for AVMs. It is a diagnostic test in which cross sections of the brain are examined through the use of x-rays and a computer. The picture of the various layers produced by the scan accurately reflects anatomic structures inside the brain, such as the ventricles, basal ganglia, and thalamus. A CT scan without contrast has a low sensitivity and is often ordered to rule out the presence of an acute cerebral hemorrhage. Calcification and infarct may be noted on these films. If the AVM has bled, serial CT scans are helpful in monitoring blood clots and observing for resolution of blood over time.
MRI/Magnetic Resonance Angiography (MRA). MRI is an initial diagnostic tool and is ordered more commonly than a CT scan for complaints such as headaches. The MRI is very sensitive, showing a homogeneous signal void on T1- and T2-weighted sequences, often with hemosiderin suggesting prior hemorrhage.20 The MRI is critical in defining the treatment plan for the AVM because it provides information about the site of the nidus, as well as its relationship to other critical anatomic structures in the brain. It is also superior to a CT scan in demonstrating subtle changes in tissue composition (e.g., edema and old hemorrhage).
MRA is the same procedure as an MRI, but the focus is on examining blood vessels instead of brain tissue. The MRA can provide some information about the presence of intranidal or feeding artery aneurysms, venous drainage patterns, and subtle AVM nidus qualities.20
Computerized Tomography Angiography (CTA). CTA is a diagnostic tool that uses x-rays to observe blood flow in arterial and venous vessels throughout the body. It is performed with a series of thin-slice axial images during injection of a bolus of IV contrast. Data are accumulated and manipulated by technicians in three-dimensions to show the blood vessels in the head or cervical region. This study may be done in addition to traditional cerebral angiography. It is unlikely that CTA will replace digital subtraction angiography (DSA) because AV shunting may only be defined by frames of the angiogram over time. CTA is a more static snapshot of the vasculature, which can help localize the lesion.
Cerebral Angiography. Cerebral angiography is the definitive diagnostic procedure for AVMs. It demonstrates the feeding arteries, the nidus, and the draining veins. This information is important for making decisions about the advisability of surgery, endovascular therapy, or radiosurgery. Cerebral angiography is also useful for following the progress and development of the AVM over time. As mentioned earlier, some AVMs are angiographically occult lesions. These lesions are discovered in the diagnostic work-up of patients with other problems such as intracranial hemorrhage or seizures.
Treatment
Decisions regarding the best approach to management of AVMs are complex. Among the factors that influence patient outcomes, the two that are critical are the reputation of the hospital and, in particular, the experience and skill of the neurosurgeon and interventional neuroradiologist with AVMs. The natural history of the
lesion for bleeding, along with the specific characteristics of the particular lesion, must be carefully weighed. The physician must compare the long-term risk presented by an untreated AVM with the more immediate risk of surgery or other treatment options.
lesion for bleeding, along with the specific characteristics of the particular lesion, must be carefully weighed. The physician must compare the long-term risk presented by an untreated AVM with the more immediate risk of surgery or other treatment options.
Most recently, controversy has arisen about the best treatment for unruptured brain AVMs. A Randomized Trial of Unruptured Brain AVMS (ARUBA) study is an international randomized trial randomizing unruptured brain AVM patients to best medical management as compared to interventional treatments. The goal of the study is to determine if treatment, by interventions such as surgery, embolization, or radiotherapy, result in better outcomes (defined as death, symptomatic stroke, and functional outcomes) than does conservative management at 5 years from discovery of an unruptured brain AVM.34 Today, AVMs are being discovered more often incidentally due to improvement in brain imaging and screening for other reasons. With more people being diagnosed, determining best treatment is important.
CASE STUDY: EL is a 34-year-old mostly Spanish-speaking woman who experienced a 10/10 headache and right lower extremity paresthesia. She has a history of migraine headaches and other headaches. A previous MRI scan revealed a left parietal AVM measuring 1 cm (AP) × 1.1 cm (TV) × 1.3 cm (SI) (Fig. 25-3). After having a baby recently, she saw a neurosurgeon who recommended an angiogram. She refused the invasive diagnostic test because of the associated risks.
Eight months after the initial diagnosis, EL presented to the emergency department, with headache and right lower extremity paresthesia. She was complaining of mild photophobia and nausea. A CTA was ordered to evaluate her known AVM. The scan revealed a left parietal intraparenchymal hematoma measuring 1.7 cm (AP) × 1.8 cm (TV) × 2 cm (SI) (Fig. 25-4). The scan also showed the presence of subarachnoid hemorrhage. The bleeding pattern and location was consistent with a hemorrhaged AVM.
The CTA helped define the anatomy of the lesion. The AVM had not grown since the initial scan. An urgent cerebral angiogram was performed to further characterize the lesion. It was reported to be a Spetzler-Martin grade I (will be described later in the chapter) AVM; the neurosurgeon decided to proceed with open surgery to remove the clot and AVM (Fig. 25-5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access