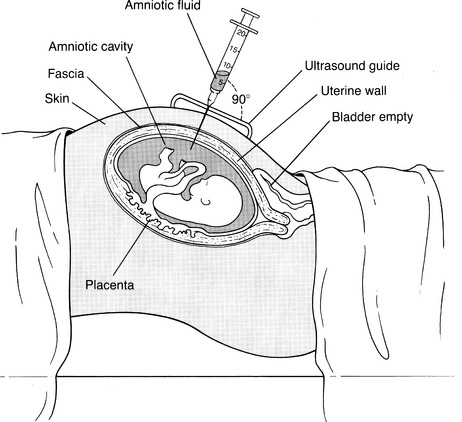
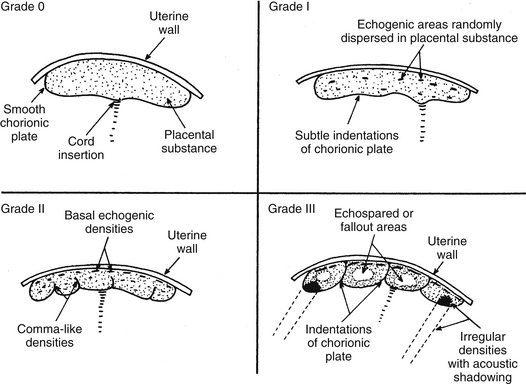
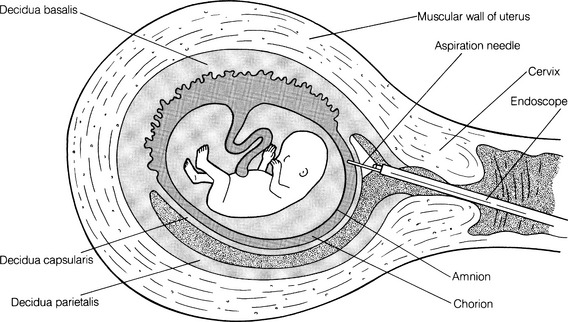
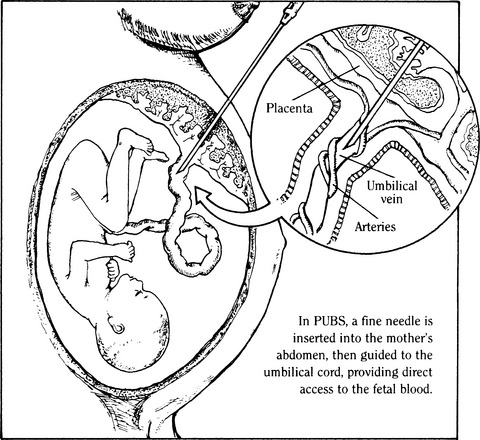
CHAPTER 8 1 Describe the various fetal diagnostic tests performed to evaluate fetal development and well-being. 2 Explain the risks and benefits of the various fetal diagnostic tests. 3 Identify high-risk pregnancy conditions that require fetal surveillance, and discuss appropriate tests for each condition. 4 List the steps in performing each test. 5 Explain the sensitivity of testing parameters for fetal well-being. 6 Describe client needs for high-risk pregnant clients undergoing a fetal diagnostic testing program and the interventions that might be helpful. Antepartum fetal assessment is an area of increasing importance today, especially as technology has grown and more studies have presented evidence-based information. It is no longer acceptable to take a “wait and see” stance when problems appear during pregnancy. Antepartum fetal assessment can be considered a twofold assessment. The first antepartum assessments occur early in pregnancy often prior to the 20th week of gestation, and generally consist of a variety of diagnostic tests to determine fetal viability, dating of the pregnancy, and/or abnormal fetal development. See Chapter 3 for additional information on fetal development. The second antepartum assessments occur later in pregnancy, after the 20th week of gestation and generally consist of a variety of diagnostic tests to determine fetal well-being or assess for fetal abnormalities. Overall, antepartum fetal assessment is based on biochemical assessment, placental grading, fetal heart rate monitoring, ultrasound biometry, amniotic fluid assessment, Doppler blood flow studies of fetal and uteroplacental circulation, and an evaluation of biophysical fetal parameters. After a pregnancy has been validated, a number of diagnostic tests may be performed to assess the viability, stability, and dating of the pregancy. As the fetus matures in utero, it begins to demonstrate a variety of fetal behaviors. If a fetus demonstrates reassuring behaviors (normal baseline fetal heart rate [FHR], normal FHR variability, presence of accelerations, and absence of decelerations, along with the presence of fetal breathing movements, fetal movements, normal amniotic fluid), the fetus is deemed “healthy” and management is usually conservative. However, in the absence of these reassuring fetal behaviors, additional fetal surveillance and/or preparation for delivery may be indicated. “Fetal behavior is an excellent diagnostic tool for evaluation of fetal well-being” (Curran & Torgersen, 2006, p. 262). Fetal behavior can be defined as any observable action or reaction to an external stimulus by the fetus (Andonotopo, Stanojevic, Kurjak, Azumendi, & Carrera, 2004; Harman, 2009). Normal fetal behavior can be equated to a normal healthy fetus. Functional development of the fetal brain begins as early as 8 to 10 weeks’ gestation, also known as the late embryonic period. With the institution of three-dimensional (3D) and 4D ultrasound, fetal behavior has become more easily tracked in utero. Between 9 and 12 weeks’ gestation, fetal movements are characterized by brisk positional and postural changes. Between 13 and 16 weeks’ gestation, the changes of position become more prolonged and include flexion and extension of the fetal limbs. Fetuses between 17 and 20 weeks’ gestation make slow flexion and extension movements of the trunk, sometimes accompanied by movement of a single limb. By the time the fetus reaches 18 to 20 weeks’ gestation, he/she performs slow, supple, and harmonious movements with isolated leg movements (Andonotopo et al, 2004; Harman, 2009; Kurjak et al, 2005). During the 9 months of gestation, fetal activities constantly expand, which correlates precisely with the development of fetal structures and the maturation of the fetal central nervous system (Morokuma et al 2004). The organization of behavioral states during the last weeks of pregnancy demonstrates that the connection between the fetal cerebral cortex and the fetal periphery is established (Blackburn, 2007). In addition, this organization demonstrates that the fetal cerebral cortex takes control over fetal activity. As a result, the fetus possesses the ability to perceive and process external signals (Harman, 2009; Morokuma et al, 2004). The majority of fetal deaths occur prior to the onset of labor. These deaths can be attributed to uteroplacental insufficiency, a state of poor perfusion at the maternal-fetal interface. Therefore, antenatal testing needs to include fetal and placental evaluations (Curran & Torgersen, 2006). In every pregnancy, basic fetal assessment is conducted at every prenatal visit by assessing for fundal height, presence of fetal movement, and auscultation of the fetal heart rate. “Antenatal testing is not routine” (Curran & Torgersen, 2006, p. 262). Assessing a fetus during the antenatal period should be used to help identify the “at risk” fetus, one that is at risk for disrupted fetal oxygenation. In the high-risk pregnancy, in which the risk of fetal morbidity and mortality can increase, fetal assessment becomes more specialized. The information gleaned from these tests and procedures helps the perinatal provider identify the risk, whether high or low, and manage the pregnancy better by weighing the risks of delivery versus the benefits of the fetus remaining in the uterine environment. In essence, antenatal testing gives the perinatal provider a way to see how the fetus is tolerating living in the intrauterine environment and how the placenta is functioning. The American Academy of Pediatrics (AAP) and American College of Obstetricians and Gynecologists (ACOG) (AAP & ACOG, 2007; ACOG, 1999) list some of the more common high-risk conditions of pregnancy that include (but are not limited to) the following: b. Antiphospholipid antibody syndrome c. Poorly controlled hyperthyroidism d. Hemoglobinopathies (hemoglobin SS, SC, or S-thalassemia) f. Systemic lupus erythematosus 2. Pregnancy-related conditions a. Hypertensive disorders of pregnancy (preeclampsia, superimposed preeclampsia) c. Hydramnios (oligohydramnios or polyhydramnios) e. Type 2 diabetes or gestational diabetes f. Preterm premature rupture of membranes a. Intrauterine growth restriction b. Rh isoimmunization (moderate to severe) c. Multiple gestation (especially with significant growth discordance) 4. Genetic assessment: may be done for clients of certain ethnic origins or with a personal or family history of genetic defect. Although there is no optimum gestational age to begin antenatal testing, most fetal surveillance for at-risk clients begins about 32 to 34 or 36 weeks’ gestation but may begin as early as 26 to 28 weeks with some high-risk conditions (ACOG, 1999; Society of Obstetricians & Gynaecologists of Canada [SOGC], 2007). For genetic concerns, assessment can begin as early as the first trimester. In summary, there are two goals of antepartum testing: (1) to identify fetuses that are at risk for permanent injury or death due to disrupted oxygenation, and (2) to identify fetuses that are “healthy,” thus preventing the use of unnecessary intervention (ACOG, 1999; Curran & Torgersen, 2006; Tucker, Miller, & Miller, 2009). In assessing the fetus during the antenatal period, it is important to consider the behavioral states of the fetus. Four behavioral states have been identified (Druzin, Smith, Gabbe, & Reid, 2007; Harman, 2009; Richardson & Gagnon, 2009). 1. State 1F (fetal)—characterized by quiescence (i.e., occasional brief gross body movements); eye movements are absent; FHR is stable with narrow oscillation bandwith 2. State 2F—characterized by frequent gross body movements, eye movements are present continually; FHR has a wider oscillation bandwidth; frequent FHR accelerations with fetal body movements 3. State 3F—characterized by zero gross body movements; eye movements are present continually; FHR stable with wider oscillation bandwidth than state 1F 4. State 4F—characterized by frequent and vigorous gross body movements; eye movements are present continually; FHR unstable with large and prolonged acceleration 5. Non–rapid eye movement (NREM; quiet sleep) and rapid eye movement (REM; active sleep) compare directly with states 1F and 2F (Richardson & Gagnon, 2009). It is important for the nurse to understand fetal growth and development. This understanding will greatly assist in his or her role during myriad antenatal assessments (see Chapter 3 for more detailed information on fetal growth and development). A The normal length of gestation for full fetal development is 280 days (40 weeks) from the first day of the mother’s last menstrual period (LMP) or 266 days (38 weeks) from actual conception; because the conception date is usually not known, the delivery date given to the mother is the estimated date of confinement (EDC), with a range of plus or minus 2 weeks to account for variations in time of ovulation from LMP. B With unknown LMP, an estimation of gestational age (EGA) can be determined by estimating fetal size; one method is to measure the distance from the upper aspect of the maternal symphysis pubis to the top of the uterus (fundal height). 1. Given a normal uterus, normal amniotic fluid volume, and a nondiabetic singleton gestation, a 20-week fetus usually causes a fundal height of 20 cm, with a normal growth rate of 1 cm per week until 36 weeks, after which engagement of the presenting part may occur. 2. If a discrepancy is found in the size-for-dates estimate, ultrasonography can be performed, preferably in the first or second trimester, a time that demonstrates the most steady growth rates. (1) The gestational sac can be measured and visualized during the first 13 weeks; measurements provide an estimated gestational age with plus or minus 9 days’ accuracy (Robinson, 1980). (2) Crown-rump length (CRL) also can be evaluated and has been accurate in 95% of cases within plus or minus 4.7 days (Box 8-1). (1) Biparietal diameter is among the most accurate second trimester measurement for determining gestational age providing a 95% accuracy within 10 to 14 days (Brookside Associates, 2008). After 30 weeks a significant difference to the altered growth rates may be found in the small-for-gestational-age (SGA), average-for-gestational-age (AGA), and large-for-gestational-age (LGA) fetuses seen in the graphs demonstrating intrauterine growth curves of weight, head growth, and length developed by Lubchenko in 1963 (Robinson, 1980). 3. Human intrauterine growth charts are clinically useful in assessing adequate serial fetal growth; the normal fetus grows from a weight of 2 to 4 g (0.1 to 0.12 ounce) and less than 2 to 3 cm (1 inch) at the onset of the fetal period (the beginning of the ninth week), up to an average weight of 3000 to 3600 g (6 pounds, 10 ounces to 7 pounds, 15 ounces) and a length of 48 to 53 cm (19 to 21 inches) at term. C Fetal organ growth is not synchronous; fetal systems grow in staggered periods. 1. Because all body organ systems are present at least in rudimentary form by the end of the embryonic stage (8 weeks), the fetal period involves tissue and organ specialization and growth accompanied by changes in body proportions. 2. Depending on their level of development, the systems have a varying degree of susceptibility to malformations caused by environmental agents and maternal conditions. 3. The fetal heart is usually large enough at 18 to 20 weeks’ gestation to be audible with a DeLee stethoscope or 12 to 14 weeks’ gestation with a Doppler ultrasound. This finding is another confirmation of gestational age. D Viability can be defined in terms of ability or capacity of a product of conception to survive for a finite time in a defined environment. Viability by law is defined as when a baby can survive for an indefinite period outside the womb with natural or artificial life-support measures. The upper limit of fetal viability is 36 weeks’ gestation plus 6 days (Behrman & Butler, 2007). The lower limit of viability is determined by fetal organ development and the current advancements in high-risk obstetric and neonatal care (Behrman & Butler, 2007). 1. Although infants born at 22 weeks’ gestation have been known to survive, most authorities believe that 23 weeks is the time of earliest survival. 2. Some systems are more immediately critical than others for survival; the respiratory system is critical because gas exchange must occur even if assisted ventilation is used. a. Pulmonary maturity generally is not achieved until approximately 37 weeks’ gestation. b. Surfactant—the substance that prevents the collapse of the alveoli—increases significantly at the 34th week; the absence of surfactant contributes to respiratory distress, a common condition of prematurity. Surfactant is stored in lamellar bodies, discharged into the alveoli, and carried into the amniotic cavity and pulmonary fluid (Leung & Lai, 2008). c. Phospholipids make up most of surfactant, the most common being lecithin and the second most common being phosphatidylglycerol (PG). d. The presence of lecithin increases significantly at about the 35th week of gestation. e. PG appears at 35 weeks’ gestation and increases rapidly between 37 and 40 weeks. In the presence of hypoxemia, the fetus uses a variety of compensatory mechanisms (Curran & Torgersen, 2006). One of the first compensatory mechanisms is to increase its oxygen supply. The fetus can do this in several ways: increase the baseline FHR, increase the hemoglobin concentration, improve cardiac contractions, and/or increase fetal oxygen extraction (Armour, 2004; Curran & Torgersen, 2006; Harman, 2009). A second compensatory mechanism the fetus uses is to control its oxygen resources by redistributing cardiac output; shunting blood to support the vital organs such as heart, lungs, brain, adrenals, and placenta; and shunting blood/oxygen away from fetal periphery or nonvital organs (i.e., stomach, colon). This redistribution is increased two- to threefold and is known as “brain sparing”—essentially keeping the brain oxygenated and alive while limiting oxygen supply to the peripheral organs and limbs (Blackburn, 2007; Curran & Torgersen, 2006). The result is decreased fetal movement while maintaining a normal pH, normal neurologic function, and normal cardiac efficacy (Blackburn; Curran & Torgersen). The final mechanism the fetus uses is to decrease oxygen consumption by decreasing FHR accelerations (or reactivity), decreasing fetal movement, and/or decreasing FHR variability (Blackburn; Curran & Torgersen; Harman, 2009). Once the compensatory mechanisms are used, the hypoxemic situation needs to be resolved (i.e., eliminate or decrease decelerations or provide supplemental oxygen to the mother, or stopping uterine activity), or the compensatory mechanisms will become depleted. Once depleted, the fetus begins to demonstrate signs of metabolic acidemia as evidenced by an increase in lactic acid in the bloodstream, cardiac dysfunction, and a progressive acidosis. If the metabolic acidemia is not corrected, permanent fetal injury can occur because lactic acid is a potent acid and severe asphyxia can cause swelling in the brain and eventually rupture the cells (Myers, Beard, & Adamson, 1969). Antepartal testing of the fetus provides a way to assess fetal oxygenation, intrauterine environment, and placental function (Blackburn; Curran & Torgersen). 1. Protein produced by the fetus. Its presence can identify fetuses that may be at risk for problems during the pregnancy. 2. However, high levels do not always indicate a problem. They can be indicative of normal and abnormal conditions such as the following: a. A fetus with a neural tube defect of the brain or spinal cord b. A baby with a birth defect of the abdominal wall c. Multiple pregnancy (more than one fetus) d. Pregnancy complications, to include miscarriage, discordant growth or death of the fetus, and placental abruption 3. If blood tests detect an abnormal alpha-fetoprotein level in a pregnant woman, an ultrasound is usually done to: a. Confirm the length (in time) of the pregnancy. b. Determine if there is more than one fetus present. c. Determine fetal status (fetal death; discordant growth). d. Detect the presence of birth defects (visual inspection and/or chromosomal studies). 4. Amniotic fluid can also be assessed for acetylcholinesterase. The presence of acetylcholinesterase, when combined with a high alpha-fetoprotein, is indicative of an increased risk of a neural tube defect. 5. The presence of alpha-fetoprotein, with or without acetylcholinesterase, indicates a neural tube defect and other abnormalities located in the esophagus (i.e., esophageal atresia) and the abdominal wall (i.e., gastroschisis). 1. Triple and quad screening is usually done around 15 to 20 weeks’ gestation. 2. This screening assesses estriol and beta-human chorionic gonadotropin (hCG) levels in addition to alpha-fetoprotein and inhibin A. 3. Triple screening includes alpha-fetoprotein, estriol, and beta-hCG. 4. Quad screening includes alpha-fetoprotein, estriol, beta-hCG, and inhibin A. 5. If these levels are elevated it could indicate an increased risk of chromosomal abnormalities (i.e., Down syndrome). Quad screening results are “positive” (abnormal) in as much as 80% of Down syndrome cases (Dungan & Elias, 2003). 1. Some pregnancies can be complicated by alloimmunization when the mother’s ABO and/or Rh is different from the fetus’s. This mismatch of blood can lead to a variety of complications from jaundice to fetal death secondary to severe fetal anemia. Essentially, this mismatch leads to a breakdown of red blood cells (RBCs) through hemolysis. This hemolysis causes an accumulation of bilirubin (a byproduct of RBC breakdown). The higher the bilirubin level, the more severe the hemolysis. If diagnosed early in pregnancy, and treated with lifesaving blood transfusion via the umbilical cord, 90% of these fetuses can survive (Oepkes et al, 2006) 2. Dr. Liley, in 1961, was the first to propose using a sample of amniotic fluid to measure the deviation of optical density at 450 nm, commonly referred to as (delta OD 450). This measurement would be used to predict life-threatening fetal anemia in the third trimester (Sikkel, et al, 2002). 3. This test was initially used solely in the first trimester. However, when intrauterine transfusion, via percutaneous umbilical cord sampling (PUBS), became a relatively safe procedure as early as 18 weeks, the procedure was also used in the second trimester to predict fetal anemia (Figure 8-1) (Oepkes et al, 2006). 4. However, amniocentesis is not without its problems, which include risk of membrane rupture, infection, worsening sensitization, and fetal loss. Some have suggested the use of Doppler ultrasound to achieve the same results as the delta OD 450 (Oepkes et al, 2006). 5. Following a number of studies conducted between 2002 and 2006, it has been determined that Doppler measurement of the peak velocity of systolic blood flow in the middle cerebral artery can safely replace invasive testing in the management of Rh-alloimmunized pregnancies. (Oepkes et al, 2006; Sikkel et al, 2002). 1. With the advent of imaging via ultrasound, tissue can be assessed in either static or real time; a wide variety of information can be gathered pertinent to maternal and fetal issues in low- and high-risk pregnancies. 2. Ultrasonography is a method of tissue imaging based on graphic analysis of the spectral characteristics of reflected high-frequency sound waves (Sonek & Nicolaides, 1998). (1) Most scanning in obstetrics is done with 3.5- and 5-MHz transducers. (2) The transducer contains crystals that emit ultrasound wave energy; it also receives the reflected sound energy as echoes. 4. Safety concerns: dosage levels a. The ratio between emitting and receiving time with diagnostic ultrasonography is only 1:1000. (1) Total exposure time during 24 hours is less than 84 seconds. (2) Therefore a fetus that is 8 cm from the source receives an average of 0.01 to 0.03 mW per square centimeter (mW/cm2), which is only 0.01% of the maximal safe level of 100 mW/cm2. b. More than 25 years of follow-up by the American College of Radiology have shown no adverse effects of diagnostic ultrasonography. a. Abdominal and vaginal ultrasound can be used. b. Advantages of vaginal ultrasound include the following: 6. Assessment: obstetric ultrasound can assess the following (Torgersen, 2005) (1) Gestational dating and/or confirmation of pregnancy (2) Diagnosis of ectopic pregnancy (3) Placental evaluation and localization (4) Diagnosis of multiple gestation (5) Guidance tool for obstetric tests such as chorionic villus sampling (CVS) b. Second and third trimesters (1) Fetal presentation and position (2) Placental evaluation for abnormalities associated with bleeding (i.e., placenta previa) (3) Confirmation of fetal viability (4) Evaluation of amniotic fluid volume or fetal well-being (i.e., biophysical profile [BPP]) (5) Survey of fetal anatomy for gross anomalies (6) Guidance tool for obstetric tests (i.e., PUBS or amniocentesis) a. Gestational dating and confirmation of pregnancy (1) Because clients in some clinic populations have questionable menstrual histories, a more accurate method for determining EDC than that based on LMP was needed. (2) This is especially true in high-risk pregnancies in which the fetal maturity estimate weighs heavily in the risk-benefit decision in planning a delivery. (3) In 1985, Queenan developed the scoring system given in Table 8-1 to identify a term fetus. TABLE 8-1 ∗See the related findings discussions for explanation of each parameter. (4) Using measurements of various parts of fetal anatomy according to the trimester, Sabbagha (1978) and others developed equations and reference tables for serial size and age determinations. (5) Fundal height measurement to assess uterine size provides a subjective assessment; however, ultrasound provides a more precise determination of gestational age. Gestational age assessment is accurate to 3 to 4 days when completed between 14 and 22 weeks’ gestation (Abuhamad, 2007). (6) Three-dimensional (3D) ultrasound can offer improved assessment of fetal growth and fetal weight (Torgersen, 2005). b. Diagnosis of ectopic pregnancy (1) Normally a fertilized egg travels down the fallopian tube, leaves the tube, and implants in the lining of the uterus. However, in about 1.9% of reported pregnancies, the fertilized egg implants in the fallopian tube. (2) Endovaginal ultrasound can be used to detect an intrauterine pregnancy. However, if unable to detect an intrauterine pregnancy with the endovaginal ultrasound, serum hCG levels are assessed. When serum hCG levels reach 1100 to 1500 mIU/mL, it can strongly suggest an abnormal gestation or ectopic pregnancy (Lozeau & Potter, 2005). (1) Grading criteria: In 1979, Grannum, Berkowitz, and Hobbins reported on a method of categorizing maturation into grades 0 to III. (a) This method was based on the identification and distribution of calcium deposits within the placenta and the increasing delineations with maturity, as in the appearance of the basal and chorionic plate of placenta and the placental substance (Figure 8-2). (b) Clinical implications (Grannum, Berkowitz, & Hobbins, 1979; Schuler-Maloney & Lee, 2004) • Straight, smooth, dense, unbroken chorionic plate • No distinct echogenic areas within the placental parenchyma • Smooth transition of the basal plate • Indicative of immature placenta; seen in the first and second trimesters of pregnancy • Well-defined unbroken chorionic plate with undulations • Multiple echogenic areas in the parenchyma • No changes in the basal plate • Seen as early as 30 to 32 weeks’ gestation; associated with about 67.7% fetal lung maturity • Deeper, more numerous indentations of the chorionic plate • Larger and more numerous echogenic areas that appear to be contiguous with the chorionic plate indentations • Larger and more dense linear echogenic areas parallel to the basal plate • Indentations of the chorionic plate extend to the basal layer with central echo-free areas. • Adjacent dense irregular echogenic areas up to 2 cm • Basal plate echogenic areas are larger, more dense, and more confluent than those in the parenchyma • Placentas more than 36 weeks’ gestation or presence of hypertension or growth-restricted fetus; this grade was associated in one study with 100% fetal lung maturity (Grannum et al, 1979) and in another with 93% lung maturity (Harman, Manning, Stearns, & Morrison, 1982). • Placenta of more than 42 weeks’ gestation; this grade has 40% incidence of villous changes, which can reduce blood flow leading to fetal hypoxia (e.g., increased calcification, intervillous thrombosis, perivillous fibrin [Eden, 1990]). (a) In the presence of vaginal bleeding episodes, diagnostic ultrasonography is used to screen for low-lying placenta or placenta previa (implantation partially or completely over the cervical os). (b) Placental migration is a term widely used to describe a placenta that appears to be “low-lying” in the first trimester and then is resolved by the third trimester. However, the term migration is a misnomer because the placenta does not move. Instead, it grows toward a better blood supply at the fundus (referred to as trophotorpism). This leaves the distal portions of the placenta to atrophy. Therefore, as the uterus continues to grow so does the lower uterine segment, giving it the appearance that the “low-lying” placenta “migrated” up to a more fundal position (Hull & Resnick, 2009). (1) Risk of discordant growth: Multiple pregnancies can be high risk, especially if monozygotic. (a) The third-trimester presentation of twin fetuses determines the mode of delivery; if vertex-vertex, many practitioners consider a vaginal birth to be safe. (b) If the first twin is vertex, some may do an external cephalic version (ECV) of the second transverse twin after the first has been born vaginally. (c) Cesarean birth is the preferred delivery method for all other presentations. (3) Clinical implication: Serial ultrasonic antepartum assessment of multiple pregnancies for fetal weight and growth discordance should start at approximately 18 weeks, with assessment occurring every 3 to 4 weeks. In the presence of fetal growth discordance or growth restriction of more than 20%, ultrasonic assessment should increase to every 2 weeks. Additionally, Doppler velocimetry may be used to further evaluate fetal well-being in multiple gestations (Malone & D’Alton, 2009). e. Guidance tool for obstetric tests such as CVS (1) CVS is a screening test taken during early pregnancy in which a small piece of placental tissue (chorionic villi) is removed from the uterus. The placental tissue is sent to the lab to assess for genetic defects. (2) CVS can be done through the cervix or through the abdomen. Ultrasound is used to find the safest approach to gather the chorionic villi specimen and to guide the medical provider to the right location. (3) Initially an abdominal ultrasound is performed to assess the position of the uterus, evaluate the size of the gestational sac, and assess the position of the placenta within the uterus (Figure 8-3). (4) If done via the cervix (transcervical), a thin plastic tube is inserted through the vagina and the cervix to reach the placenta. The ultrasound is used to help guide the plastic tube to the appropriate area. Then a small sample of chorionic villi tissue is removed. (5) If done via the abdomen (transabdominal), a needle is inserted through the abdomen and uterus and into the placenta. The ultrasound is used to help guide the needle to the appropriate area. Then a small sample of chorionic villi tissue is removed. f. Fetal presentation and position (1) Vertex: Malpresentations have a much higher incidence of morbidity associated with vaginal birth than do cephalic presentations; identifying the fetal presentation in labor accurately is crucial; visualization by ultrasonography of the fetal skull outline at the maternal pelvic brim is a reassuring finding. (2) Breech: Breech presentation in the last trimester may be manipulated into a vertex presentation by an external cephalic version (ECV) procedure; antepartum assessment of presentation can also be of value in enabling a timely intervention to avoid a malpresentation at delivery. (3) Transverse: Just as with breech presentation, transverse presentation in the last trimester may be manipulated into a vertex presentation by an ECV, and ultrasound evaluation can provide timely intervention to avoid a malpresentation at delivery. (4) The position of the fetus plays an important role in determining the course of labor. It also plays an important part in whether the fetus will fit through the maternal pelvis (see Chapter 10 for additional information on fetal positions). (a) The most common position for birth is with the fetus’s head positioned down, facing the mother’s back, with the fetal chin tucked to its chest. This position allows the back of the head (smallest diameter) to enter the pelvis. Most fetuses settle into this position between 32 and 36 weeks’ gestation. (b) Occasionally the fetus is positioned with the head down as it should be, but it is facing the mother’s abdomen. This position is referred to as occiput posterior and can increase the chance of painful and prolonged delivery because the softer part of the fetal head is presenting first, trying to dilate the cervix. g. Placental evaluation for abnormalities associated with bleeding. See Chapter 21 for additional information on hemorrhagic disorders. (1) Although the majority of women presenting with second- or third-trimester vaginal bleeding may experience minimal blood loss, it is still a situation that needs immediate evaluation. Ultrasound is a useful tool to assess the degree and potential cause of vaginal bleeding (Hertzberg, 2007). (2) Bleeding in the second and third trimesters can be attributed to a variety of conditions including, but not limited to, placenta previa, abruptio placentae (placenta separates from the uterine lining), placenta cretas (placenta invades into the uterine lining and/or the uterine musculature), uterine rupture (uterus develops a hole in the uterine musculature with the fetus/fetal parts extruding into the peritoneal cavity), or vasa previa (placental or umbilical cord blood vessels are trapped between the fetus and the opening to the birth canal) (Hertzberg, 2007). h. Confirmation of fetal viability (1) Fetal life can be confirmed by the visualization of the heart’s beating and of fetal movements. (2) Real-time ultrasonography provides a fast and effective method of assessing intrauterine fetal demise (Merz, 2007). i. Evaluation of amniotic fluid volume or fetal well-being (a) Most researchers agree that less than 500 mL (or amniotic fluid index [AFI] <5 cm) of amniotic fluid at term is considered oligohydramnios, or decreased fluid, and more than 2000 ml (or AFI >25 cm) is considered polyhydramnios (Beall & Ross, 2009; Brace & Wolf, 1989). [i] The AFI is an index developed by Rutherford, Phelan, Smith, and Jacobs (1987) in which the depths of amniotic fluid measured in all four quadrants surrounding the maternal umbilicus are totaled. The depths are described in centimeters. [ii] Interpretation currently recommended is based on findings of an increased perinatal morbidity (low Apgar scores, meconium staining, fetal distress) in pregnancies with lower-than-normal measurements at term (Richards, 2007). (2) Fetal well-being: It is important to be able to assess the fetus during the course of pregnancy, especially in light of underlying maternal medical or obstetric problems (i.e., hypertensive disorders; diabetes mellitus) that can have an effect on the fetus. Ultrasound can be used alone or is often combined with other assessment tools to determine the status of fetal well-being. Assessment tools using ultrasonography include the biophysical profile, modified biophysical profile, 3D or 4D ultrasound, and Doppler velocimetry. Further descriptions of these assessment tools are described under Ancillary Antenatal Tests (see section B, following). j. Survey of fetal anatomy for gross anomalies: Congenital anomalies and follow-up directed (level-2) scans. See Chapter 2 for further information on genetics and Chapter 17 for further information on congenital abnormalities. (a) Based on 2007 Centers for Disease Control and Prevention (CDC) data (CDC, 2007), approximately 1% to 2% of live-born infants have a major anomaly. (b) At least 500 known developmental anomalies have been discovered. (c) Between 6% and 11% of all stillbirths and neonatal deaths are the result of aneuploid fetuses; morbidity as the result of chromosomal defects accounts for another 0.65% of newborns (ACOG, 2007a). (d) Testing, using integrated tests from the first and second trimesters, has a 92% to 96% detection rate and a 5% false-positive rate for Down syndrome (Barclay, 2008). (e) The recognition of an anomaly may influence the location and method of delivery so that neonatal outcome may be optimized (CDC, 2007). (f) Nuchal lucency has been added to the screening tests for Down syndrome, done between 11 and 14 weeks’ gestation; congenital anomalies and heart disease, along with Down syndrome, have been associated with the increase in size of the normal clear area posterior to the fetal neck (ACOG, 2007a; Dungan & Elias, 2003). (2) Directed scans are performed as a thorough examination of a client suspected of carrying a physiologically or anatomically defective fetus, based on her history, clinical evaluation, or previous ultrasonography. (3) Management of anomalies depends on consideration of variables such as: k. Guidance tool for obstetric tests, such as amniocentesis and PUBS (1) Amniocentesis for determination of fetal lung maturity (AAP & ACOG, 2007) (a) Lecithin/sphingomyelin (L/S) ratio: The chance of lung maturity is 98% if the concentration of lecithin is twice that of sphingomyelin in lung surfactant secreted by the fetus into the amniotic fluid (L/S ratio greater than 2:1) in the nondiabetic client. [i] Lecithin is key to the formation and stablization of the active surface layer that prevents the pulmonary alveoli from collapsing (Schuler-Maloney & Lee, 2004). [ii] Sphingomyelin is a membrane lipid present in amniotic fluid. It is not related to fetal lung maturity (Schuler-Maloney & Lee, 2004). [iii] L/S ratio 1:1: before 32 to 34 weeks’ gestation [iv] L/S ratio 2:1: as early as 35 weeks’ gestation [v] In the presence of maternal diabetes, fetal acidosis, or fetal sepsis, the L/S ratio can be favorable, yet still result in an infant with respiratory distress syndrome (Mercer, 2009; Schuler-Maloney & Lee, 2004). See Chapter 22 for further discussion on testing in diabetic women. [i] PG is one of the last pulmonary phospholipids to be evident in amniotic fluid, usually appearing after 35 weeks’ gestation. [ii] PG is highly predictive of fetal lung maturity in diabetic clients, or in specimens contaminated with blood or vaginal fluids. [iii] In addition to the L/S ratio, the presence of PG is required for definitive maturity assessment. [i] Lamellar bodies are surfactant-containing particles secreted by type II pneumocytes. Lamellar bodies carry surfactant, are the same size as platelets, and can be counted via the same laboratory equipment that performs a platelet count (e.g., Coulter counter). They are found in the amniotic fluid. The number of lamellar bodies increases with the onset of functional fetal lung maturity (Mercer, 2009). [ii] The test requires 1 mL of amniotic fluid and takes less than 15 minutes to complete (Greenspoon, Rosen, Roll, & Dubin, 1995). [iii] An LBC >50,000/μL is highly predictive of pulmonary maturity (Mercer, 2009). (d) An LBC <15,000/μL is highly predictive of pulmonary immaturity (Mercer, 2009). (e) The following can interfere with LBC results: [i] Meconium; LBC not recommended with meconium (Mercer, 2009) [ii] Vaginal bleeding. If vaginal bleeding is present, a hematocrit should be performed. If the hematocrit is more than 1%, notify the primary health care provider because an elevated hematocrit will falsely elevate LBC results (Mercer, 2009). [iii] Vaginal mucus can disrupt the counters leading to a decreased count (Mercer, 2009). [iv] Hydramnios: Severe oligohydramnios can falsely increase the LBC (indicating lungs are mature), whereas polyhydramnios can falsely decrease the LBC (indicating the lungs are immature) (Mercer, 2009). (2) PUBS (Dungas & Elias, 2003) (a) Used after ultrasound has detected an anomaly in the fetus (b) Used when a rapid chromosome analysis is needed, especially if analysis is needed toward the end of the pregnancy. Results are usually available within 48 hours, depending on the laboratory process. (c) The procedure is conducted much like an amniocentesis; under ultrasonography, the provider inserts a needle through the abdominal wall. However, instead of the needle staying in a pool of amniotic fluid, it is guided to insertion into the umbilical cord (Figure 8-4). (d) A sample of the fetal blood is then withdrawn and sent to the laboratory for analysis. (e) Loss of pregnancy with this procedure is approximately 1 in 100 pregnancies. (f) Clients, and their fetuses, undergoing amniocentesis and PUBS are not without risk. For example, there is risk for maternal-fetal injury and risk for infection. Clients should be provided with a simple, clear explanation of each procedure that includes the purpose of the test, the frequency of the test, the risks, the benefits, and complications of the procedure. A Allow the client to vent her frustrations with the discomfort, time-consuming demands, and limitations imposed by this high-risk pregnancy and fetal surveillance program. B Assist the client in setting realistic goals for progress in her pregnancy so that she experiences a sense of accomplishment. 1. Each day in utero is beneficial to the normal development of her fetus. 2. The fetus has improved chances for survival as long as the testing remains reassuring. 3. Compliance with treatment and testing regimens is beneficial to the fetus. 1. Use sterile technique to prevent chorioamnionitis. 2. Position client properly (in lateral or wedged position) to avoid supine hypotension during fetal monitoring tests. 3. Administer local anesthesia when starting an IV, as permitted by the institutional protocol. 4. Use comforting measures such as touch during difficult procedures. 5. Explain procedures step by step. 6. Encourage the client’s significant other to be with her if she requests it during procedures. 7. Have terbutaline, 0.25 mg, available for subcutaneous (or IV) injection for uterine contractions, should they occur. 8. Schedule testing appointments at the client’s convenience when possible. 9. Follow up when the client does not keep an appointment. Reinforce the value of testing. As early as 8 to 9 weeks gestation, the fetus begins to move, to kick, and to stretch; however, maternal perception of her fetus moving is often delayed until 16 to 20 weeks’ gestation (ACOG, 1999; Curran & Torgersen, 2006). In all pregnancies, fetal movement is a sign of fetal well-being. In high-risk pregnancies the fetus may be at an increased risk for altered perfusion, that is, a redistribution of the fetal cardiac output. Fetal movement (FM) is an inexpensive and noninvasive method of screening that is often taught to high-risk pregnant women (Grant, Elbourne, Valentin, & Alexander, 1989; Harman, 2009). In low-risk pregnancies, perception of FM is discussed at every prenatal visit, and women are told to inform their health care provider or to call/go to Labor and Delivery if there is a decrease in FM or if no FM is felt. FMC is also referred to as fetal kick counting or kick counts (ACOG, 1999; Atterbury, Mikkelsen, & Santa-Donato, 2003; Curran & Torgersen, 2006). a. Decreased activity in a previously active fetus may reflect disturbance of placental function and may be a clue to impending demise (Sadovsky, 1985a). A sudden total cessation of fetal movement is definitely alarming and may signify a premortem event and not just a “warning” of impending fetal compromise (Harman, 2009). b. Many variables, such as gestational age, diurnal rhythm, fetal behavior states, medications, tobacco or fetal malformation, can affect FM and should be considered when evaluating decreased fetal movement (Curran & Torgersen, 2006)
Antepartum Fetal Assessment
INTRODUCTION
Fetal Behavioral States
Fetal Growth and Development
Fetal Response to Hypoxemia
CLINICAL PRACTICE
Biochemical Assessments
Biophysical Assessment
Traits of Parameter
Maturity
Biparietal diameter (BPD)
>9 cm
Placental grading
II-III
Amniotic fluid volume (AFV)
Normal to crowding
Interventions
Ancillary Antenatal Tests
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Antepartum Fetal Assessment
Get Clinical Tree app for offline access