Chapter 31 AIDS-associated toxoplasmosis
Introduction and Epidemiology
Toxoplasma gondii is among the most prevalent causes of latent infection of the central nervous system (CNS) throughout the world. After an acute infection, cysts of T. gondii persist in the CNS and in multiple extraneural tissues. Although normal human hosts maintain infection in a quiescent state, immunocompromised individuals may be at risk for reactivation and dissemination of chronic (latent) infection. Defective T cell-mediated immunity in HIV-infected patients results in loss of the primary arm of host defense against this parasite. Reactivation of latent infection in patients with AIDS may lead to clinically apparent disease (toxoplasmosis), which most frequently manifests as life-threatening toxoplasmic encephalitis (TE) [1].
Seroprevalence varies among geographic locales and even within subpopulations of the same locale [2, 3]. The seroprevalence of the infection with the parasite in HIV-infected individuals usually reflects the seroprevalence of the population they come from. Studies performed in the United States reveal an age-adjusted seroprevalence of approximately 10% [4], with a higher prevalence (>25%) among certain ethnic groups. Early studies indicated that 20–47% of T. gondii-seropositive patients with AIDS ultimately developed TE [5]. The risk of toxoplasmosis has significantly decreased since the introduction of primary prophylaxis against T. gondii and antiretroviral therapy (ART). The incidence in the USA among patients who had developed AIDS declined from 2.1/100 person-years (PY) in 1992 to 0.7/100 PY in 1997 [6]. However, TE remains a prevalent opportunistic infection in patients with signs and symptoms of central nervous system involvement even in the late ART era, particularly among severely immunosuppressed patients whose CD4 counts decline <50 cells/mm3 and who are not taking anti-Toxoplasma prophylaxis. TE can be the presenting illness in patients with AIDS [7]. In two European studies TE still was the most prevalent central nervous system infection in HIV-infected patients during the past decade [8, 9].
Primary infection is rarely symptomatic in HIV-infected patients or in those with AIDS. However, Toxoplasma-seronegative individuals infected with HIV should be counseled about how to avoid exposure to the parasite. Ingestion of undercooked or raw meat containing tissue cysts and of vegetables or other food products contaminated with oocysts is a major means of transmission of the parasite, as is direct contact with contaminated cat feces. Although cats are the definitive host for T. gondii, antibody seroconversion in adult HIV-infected individuals appears unrelated to cat ownership or exposure [10]. Recently, drinking of unfiltered drinking water and ingestion of raw mussels, oysters, and clams have also been implicated as a source of acute T. gondii infection [11].
Clinical Presentation
AIDS patients who develop TE can do so after the diagnosis of AIDS has been made [12, 13]. Because multifocal involvement of the CNS frequently occurs in TE in AIDS patients, there may be a wide spectrum of clinical findings, including alteration of mental status, seizures, motor weakness, sensory abnormalities, cerebellar dysfunction, meningismus, movement disorders, and neuropsychiatric manifestations [14]. The characteristic presentation is usually one of subacute onset, with focal neurologic abnormalities in 58–89% of patients. Altered mental status, manifested by confusion, lethargy, delusional behavior, frank psychosis, global cognitive impairment, anomia, or coma, may be present initially in as many as 60% of patients. Seizures are the reason for seeking medical attention in approximately one-third of AIDS patients with TE. Focal neurologic deficits are evident on neurologic examination in approximately 60% of patients. Although hemiparesis is the most common focal neurologic finding, patients may have evidence of aphasia, ataxia, visual field loss, cranial nerve palsies dysmetria, hemichorea-hemiballismus, tremor, parkinsonism, akathisia, or focal dystonia [10]. In addition, infection of the spinal cord with T. gondii has been described in cases of transverse myelitis, conus medullaris syndrome [15], and of ventriculitis accompanied by hydrocephalus [16].
A rapidly fatal panencephalitis form of diffuse cerebral toxoplasmosis has also been described [17]. Unfortunately, computed tomography (CT) of the head was unrevealing in these cases [17, 18]. In case reports involving HIV-uninfected, severely immunocompromised patients with biopsy-proven diffuse TE, no changes were reported on magnetic resonance imaging (MRI) with gadolinium in the majority of patients, and only minimal changes were detected in one patient (e.g. minimal enhancement of the cortex and subcortical white matter) [19, 20]. Hence, although rare, diffuse TE should be suspected as a possible cause of severe encephalitis in patients with advanced immunosuppression (e.g. those with CD4 count below 50 cells/mm3) in whom other causes have been ruled out and the suspicion for TE is high despite the lack of brain-occupying lesions in MRI. Extracerebral sites with or without concomitant TE may be involved in HIV-infected individuals. As is true for TE, extracerebral toxoplasmosis usually occurs in patients with CD4 counts of <100 cells/mm3 [21–23]. In patients with extracerebral toxoplasmosis, ocular and pulmonary sites are most commonly involved (50 and 26% of patients, respectively) [10].
Significant pulmonary disease, including acute respiratory distress syndrome caused by toxoplasmosis, has been reported. Mortality, even in the presence of treatment for toxoplasmosis, is high in these patients [24]. The most common clinical syndrome is a prolonged febrile illness with cough, hypoxemia, and dyspnea that is clinically indistinguishable from Pneumocystis pneumonia. This presentation has also been reported as a manifestation of an undiagnosed HIV infection [25]. Associated extrapulmonary disease caused by T. gondii has been reported in approximately 50% of the patients at the time of clinical presentation. TE may precede or follow pulmonary toxoplasmosis if maintenance therapy is not instituted. A highly lethal syndrome of disseminated toxoplasmosis has been described in AIDS patients that present with fever and a sepsis-like syndrome with hypotension, disseminated intravascular coagulation, elevated lactate dehydrogenase, and pulmonary infiltrates [23]. This syndrome is usually not associated with clinical or radiologic evidence of TE [21].
Ocular disease caused by toxoplasmosis occurs relatively infrequently in AIDS patients (compared with the incidence of cytomegalovirus retinitis) [26]. Ocular pain and loss of visual acuity are typical complaints, and funduscopic examination typically reveals findings consistent with necrotizing retinochoroiditis. The lesions are yellow-white areas of retinitis with fluffy borders. In reported series, the lesions were multifocal in 17–50%, bilateral in 18–40%, and accompanied by optic neuritis in approximately 10% of the cases. Scant retinal inflammation is frequently observed in AIDS-associated toxoplasmic retinochoroiditis [26]. Thus the features of toxoplasmic retinochoroiditis commonly observed in the immunocompetent host may be absent in patients with AIDS. Vitreal inflammation may vary from mild localized vitreal haze to extensive vitreous inflammation. Vasculitis and hemorrhage are uncommon. In most patients the ocular lesions are located away from areas of pre-existing scars. This suggests that the pathogenesis of these lesions may be secondary to hematogenous seeding rather than local reactivation of infection. The presence of concurrent TE in AIDS patients with ocular toxoplasmosis has varied from 29 to 63% [27, 28].
Most AIDS patients with TE (80–95%) have CD4 counts of <100 cells/mm3. Cerebrospinal fluid (CSF) may be normal or reveal mild pleocytosis (predominantly lymphocytes and monocytes) and an elevated protein level, whereas the glucose content usually is normal [13].
Congenital toxoplasmosis and the HIV-infected woman
As with HIV-uninfected women, women infected with HIV are at risk for transmission of T. gondii infection to their fetus if they are seronegative for Toxoplasma and acquire infection during pregnancy [29]. In addition, maternal–fetal transmission can occur in HIV-infected pregnant women who are chronically infected with T. gondii; however, the risk of transmission is low (less than 4%) [30, 31]. Studies that addressed this problem were conducted in cohorts of primarily asymptomatic women, most of whom had a CD4 count >200 cells/mm3 [32, 33]. The risk of transmission may be higher in severely immunocompromised HIV-infected women, particularly in those in whom clinical reactivation occurs (e.g. TE) [34]. However, there are insufficient data to accurately estimate this risk. In one study, one of the three dually infected mothers with CD4 counts <100 cells/mm3 transmitted T. gondii infection to her baby [32]. When dually infected women developed toxoplasmosis during pregnancy, 75% of their infants were born with congenital toxoplasmosis and HIV infection [3]. All infants with congenital toxoplasmosis born to mothers who were HIV-infected were also infected with HIV. The initial clinical presentation of congenital toxoplasmosis in the HIV-infected infant is similar to that in HIV-uninfected infants but appears to run a more rapid and progressive course. The infants often appear normal at birth. In the ensuing months, they fail to gain weight or develop appropriately. The majority develop multisystem organ involvement, including the CNS, heart, and lungs [30].
Diagnosis
At present, the definitive diagnosis of toxoplasmosis in AIDS patients can only be made by demonstration of the organism in tissues or amplification of T. gondii DNA from body fluids (i.e. CSF, BAL, vitreous fluid, peripheral blood, urine) (Box 31.1). In cases where TE is highly likely (see Management), brain biopsy can be deferred while awaiting results of empiric anti-T. gondii therapy. The likelihood of TE is high in AIDS patients who are seropositive for T. gondii, whose CD4 count is <200 cells/mm3 (risk is greater when it is <100 cell/mm3 [35]), have multiple ring-enhancing lesions on MRI of the brain, and who are not taking anti-Toxoplasma prophylaxis. In this clinical scenario, an optimal response to anti-Toxoplasma therapy further supports the diagnosis of TE. In these patients an inadequate clinical response to treatment (e.g. lack of 50% improvement in the patient’s neurological exam by day 7) makes the diagnosis less likely and should prompt a lumbar puncture, if safe and feasible, for examination of the CSF by polymerase chain reaction (PCR), and/or a brain biopsy. The morbidity associated with brain biopsy for the evaluation of focal brain lesions in HIV-infected patients is less than that from an erroneous diagnosis [36]. Thus, a brain biopsy should be strongly considered in cases where the likelihood of TE is low if lumbar puncture is not possible or if PCR testing is non-diagnostic. In addition to TE, examination of the CSF by PCR can also be diagnostic for JCV-associated progressive multifocal leukoencephalopathy (PML), EBV-associated central nervous system lymphoma, and CMV ventriculitis. The likelihood of TE is low in Toxoplasma-seronegative patients, those with CD4 counts >200 cells/mm3, those with single space-occupying lesions on brain MRI, and those who have been compliant with anti-Toxoplasma prophylaxis [10].
• Serology (including titer in differential agglutination assay, IgG, IgMa)
• Visual demonstration of T. gondii in body fluids (CSF, BAL) by microscopic examination (i.e. using Wright-Giemsa stain)
• Amplification of T. gondii DNA by PCR examination of body fluids (i.e. CSF, peripheral blood, BAL, vitreous fluid) or tissue biopsies
• Histologic evaluation, including immunoperoxidase staining of tissue biopsies
• Isolation of T. gondii from tissue biopsies or body fluids (i.e. CSF, blood, BAL)
• CT scans and/or MR images of the brain in an attempt to demonstrate the presence of multiple ring-enhancing lesions
a Useful, mainly in areas of high seroprevalence, IgM positive results should undergo confirmatory testing at a reference laboratory (e.g. in the USA, at the Palo Alto Medical Foundation Toxoplasma Serology Laboratory [PAMF-TSL]; Palo Alto, CA; http://www.pamf.org/serology/; telephone number (650) 853-4828; e-mail toxolab@pamf.org).
Serology
Although almost all AIDS patients with TE have detectable IgG T. gondii in their serum, published series have reported a 0–3% seronegativity rate [14].
Toxoplasma gondii IgM antibodies, routinely measured in an attempt to diagnose acute toxoplasmosis in non-AIDS patients, are rarely demonstrable in AIDS patients with TE. In HIV-infected patients whose Toxoplasma serology results are unknown, both IgG and IgM should be measured initially. Positive results on both tests should suggest recently acquired infection, though this is not diagnostic of toxoplasmosis [3]. Positive or equivocal IgM results are not necessarily diagnostic of an acute infection; in fact they may be false positive or observed in chronically infected individuals. Thus, HIV-infected patients with equivocal or positive Toxoplasma IgM results should undergo confirmatory testing at a reference laboratory when feasible (e.g. in the USA, at the Palo Alto Medical Foundation Toxoplasma Serology Laboratory [PAMF-TSL]).
Isolation studies
Toxoplasma gondii readily forms plaques in tissue cultures of human foreskin fibroblasts and most other cultured cells [37, 38]. It has been isolated from the blood in 14–38% of AIDS patients with toxoplasmosis [39, 40]. Toxoplasma gondii may also be isolated from bronchoalveolar lavage (BAL) fluid in patients with toxoplasmic pneumonitis [41].
DNA detection (PCR)
The high specificity of PCR testing for T. gondii DNA makes this method of diagnosis useful when positive. The use of the PCR has enabled detection of T. gondii DNA in brain tissue [42, 43], CSF [44, 45], BAL fluid [46, 47], peripheral blood [44, 48, 49], aqueous humor [42, 46], and vitreous fluid [50] of AIDS patients with toxoplasmosis. Because T. gondii cysts persist in certain organs (i.e. brain, skeletal and heart muscle, and eyes) for years after infection, a positive PCR in these tissues does not necessarily reflect active infection.
Of note, attempts to use PCR from amniotic fluid to make a prenatal diagnosis of congenital infection have been hampered by concerns about potential of transmission of HIV to the fetus during amniocentesis [51]. However, in a recent study by Mandelbrot and colleagues, the risk of mother-to-child transmission was negligible in mothers taking ART. Thus, in HIV-infected women who acquired primary T. gondii infection during gestation or in women with AIDS who develop toxoplasmosis, amniocentesis can be performed, if they are taking effective ART [52].
Neuroimaging studies
Imaging studies of the brain are essential for diagnosis and management of patients with TE [53]. Typically, multiple, bilateral, hypodense, enhancing mass lesions are found on CT scan. Lesions have a predilection for, but are not limited to, the basal ganglia and hemispheric corticomedullary junction. Significant enhancement of intracerebral lesions is usually seen on CT scan. However, Toxoplasma abscesses may fail to enhance or may be solitary and located anywhere in the brain. MRI is more sensitive than CT scan for detection of brain lesions in patients with TE. Masses demonstrated by MRI may be absent on CT scan [54], whereas the converse is not true. Abnormal contrast-medium enhancement, both on CT and MRI, appears to correlate with the CD4 count: pathological uptake may be absent or mild with CD4 count <50 cells/mm3 and increases accordingly with increasing CD4 count [55, 56].
The neuroradiologic response of TE to specific treatment is seen on CT as a reduction in mass effect, number and extent of lesions, and enhancement. Although the time to resolution of lesions may vary from 20 days to 6 months, the vast majority of patients who respond clinically will also show radiographic improvement [57, 58]. The MRI response to therapy also varies with the location and complexity of the mass lesion. Persistent enhancement on CT scans or MRI after treatment for TE has been associated with a higher incidence of subsequent relapse. Findings on MRI and CT scans are not pathognomonic for TE. Primary CNS lymphoma cannot be distinguished from toxoplasmosis solely on the basis of neuroradiologic criteria, as both present as contrast-enhancing lesions with mass effect. However, the presence of hyperattenuation on non-enhanced CT scans, subependymal location, and crossing of the corpus callosum suggest the possibility of lymphoma [59]. Other imaging techniques appear to be useful for distinction between CNS lymphoma and infectious processes in HIV-infected patients with focal brain lesions. Magnetic resonance techniques, positron emission tomography scanning and radionuclide scanning have been used to evaluate AIDS patients with focal CNS lesions, specifically to differentiate between toxoplasmosis and primary CNS lymphoma. Magnetic resonance spectroscopy (MRS), although widely available, is not usually performed; differences between CNS lymphoma and TE in levels of lipids and lactate have been reported. However, the improper choice of voxel positions in clinical practice may have contributed to the failure to accurately distinguish differences between the spectra of lymphoma and toxoplasmosis [60–62]. Fluorodeoxyglucose [18F]-positron emission tomography (FDG-PET) is used for the diagnosis of tumors in the CNS; uptake of [18F] is significantly higher in patients with CNS lymphoma compared to patients with TE [63–66]. Although the studies reported high sensitivity and specificity for the diagnosis of CNS lymphoma, the number of patients was small, patients were receiving empirical treatment for TE, and the procedures were performed several days after treatment was initiated, which can decrease the uptake of the lesions. Similarly, several studies demonstrated increased uptake by thallium-201 single-photon emission computer tomography (201Tl SPECT) as highly sensitive and specific for the diagnosis of CNS lymphoma in HIV-infected patients [67–69]. It has also been used as a complementary imaging procedure for MRI with improved diagnostic accuracy [70]. 201Tl SPECT may have decreased diagnostic utility in HIV-infected patients receiving ART [71].
Histopathology
Definitive diagnosis of TE often requires demonstration of the organism on histopathologic section of brain tissue obtained at biopsy. Some evidence suggests the superiority of open excisional biopsy compared to needle biopsy in making the histopathologic diagnosis of TE. The response of the brain to T. gondii infection can vary from a granulomatous reaction with gliosis and microglial nodule formation to a severe focal or generalized necrotizing encephalitis [72]. Perivascular and intimal inflammatory cell infiltrates can lead to fibrosis necrosis, which can result in hemorrhage or thrombosis, accounting for neurologic signs and symptoms.
The presence of numerous T. gondii tachyzoites or cysts surrounded by an inflammatory reaction is diagnostic. Cysts or free organisms (tachyzoites) not demonstrable on routine histopathologic examination can be identified using the peroxidase-antiperoxidase method [73]. Thus, when routine histopathologic studies fail to provide a definitive diagnosis, appropriately fixed brain tissue should be stained by the immunoperoxidase technique in an attempt to identify T. gondii antigens or organisms.
Differential Diagnosis
The main differential diagnosis of HIV-infected patients with focal brain lesions is between CNS lymphoma and TE. In Toxoplasma-seropositive, HIV-infected patients with a CD4 count of <200 cells/mm3 who are not receiving anti-T. gondii prophylaxis, the presence of multiple enhancing lesions is strongly suggestive of TE. In patients with a low probability of having TE, the initial differential diagnosis should also include PML, fungal abscess (e.g. cryptococcoma), tuberculosis, pyogenic brain abscess, syphilitic lesions (gummas) [74] or cytomegalovirus disease, and Kaposi’s sarcoma in addition to TE. Because therapy is available for most of these disorders, brain biopsy for histopathologic diagnosis in patients with low likelihood of having TE (e.g. single lesion by MRI, negative anti-Toxoplasma IgG, CD4 count >200 cells/mm3, use of anti-Toxoplasma prophylaxis, lack of response to therapy) may be necessary for successful management of the patient. The characteristic appearance of PML on neuroimaging studies often facilitates differentiation of this disorder from other causes of intracerebral mass lesions. Generally, lesions are multifocal and asymmetric at presentation, predominantly involve white matter, and progress in size and number. Ring enhancement, edema and mass effect are rare [74–76] and more often seen with immune reconstitution inflammatory syndrome (IRIS) [77]. However, brain biopsy remains the gold standard for definitive diagnosis.
Management
General principles
Because TE generally reflects reactivation of a latent infection, all HIV-infected individuals should be tested for anti-Toxoplasma IgG antibody. For those who are seronegative, we recommend periodic repeat testing. Those who are seropositive are at risk for development of TE (Fig. 31.1). Studies have associated TE with a more rapid progression of HIV, high TE mortality 16–40%, and persistent neurologic impairment 40% [78, 79]. Therefore, prevention of the disease is critical.
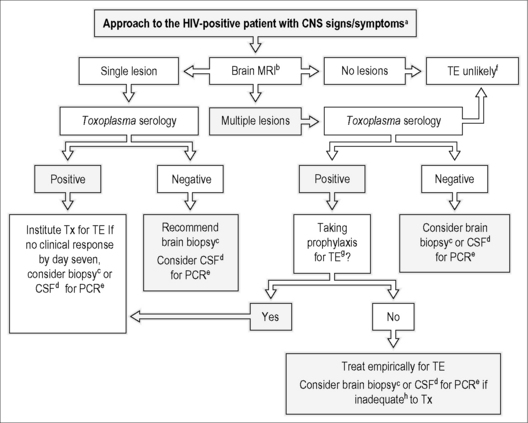
Figure 31.1 Guidelines for the evaluation and management of patients with suspected toxoplasmic encephalitis (TE).
(a) Patients with TE may present with a non-focal neurological examination. (b) MRI is superior to CT scan. (c) T. gondii-specific immunoperoxidase stain is both highly sensitive and specific for the diagnosis of TE. (d) CSF should be obtained only if safe to perform a lumbar puncture. (e) In addition to PCR for T. gondii, consider PCR for EBV (PCNSL), JCV (PML), and CMV. (f) If CD4 T count <50 cells/mm3, Toxoplasma IgG is positive, and no other cause of encephalitis has been identified, suspect diffuse TE. (g) For regimens considered to be effective for TE prophylaxis see Table 31.2. (h) Inadequate response to therapy is defined as deterioration of the neurological findings within 3 to 5 days of institution of an appropriate anti-Toxoplasma regimen or no significant clinical response (less than 50% improvement in neurological examination) within 7 to 10 days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


