Chapter 18 Acute kidney injury and dialysis
Acute kidney injury (AKI) is the contemporary name for what was formerly termed acute renal failure. The new name more accurately describes the process of all phases of acute injury to the kidney. The definition of AKI can be referred to as RIFLE, representing three levels of renal dysfunction. These levels are described in the following categories: risk of renal dysfunction, injury to the kidney, failure of kidney function, loss of function, and end-stage renal disease, the later two representing outcome categories (Table 18-1). Dialysis is often necessary for the treatment of AKI. The most common indications include uremia, hyperkalemia, acidosis, fluid overload, and drug overdose.
Table 18-1 Risk, Injury, Failure, Loss, and End-Stage Renal Diseases (RIFLE) Classification
| Class | Glomerular filtration rate criteria | Urine output criteria |
|---|---|---|
| Risk | Serum creatinine × 1.5 | < 0.5 mL/kg/h × 6 h |
| Injury | Serum creatinine × 2.0 | < 0.5 mL/kg/h × 12 h |
| Failure | Serum creatinine × 3 | < 0.5 mL/kg/h × 24 h, or anuria × 12 h |
| Loss | Persistent acute renal failure = complete loss of kidney function > 4 weeks | |
| End-stage renal disease | End-stage renal disease > 3 months |
For conversion of creatinine expressed in conventional units to SI units, multiply by 88.4. RIFLE class is determined based on the worst of either glomerular filtration criteria or urine output criteria. Glomerular filtration criteria are calculated as an increase of serum creatinine above the baseline serum creatinine level.
From Clarkson MR, Brenner BM, Magee C: Pocket companion to Brenner and Rector’s the kidney, ed 2, St. Louis, 2010, Saunders.
What are the types of acute kidney injury?
AKI is divided into three categories: prerenal, intrarenal, and postrenal (Box 18-1) (see Chapter 4).
Prerenal. Prerenal renal failure accounts for approximately 70% of AKI cases. Prerenal events result in a decrease in blood flow to the kidney. Examples include congestive heart failure, hypovolemia, sepsis, myocardial infarction, prolonged hypotension, and vascular disorders of the renal artery or vein.
Intrarenal. Approximately 25% of AKI cases are caused by intrarenal factors. Any event that damages the kidney tissue, structure, and function is categorized as intrarenal AKI. The damage, which may involve the glomeruli, the tubules, or both, interferes with the ability of the kidneys to carry out their normal functions. The most common cause of intrarenal failure is damage to the tubules. This is called acute tubular necrosis (ATN). ATN is caused by severely reduced blood flow leading to prolonged ischemia, or by direct toxic insult to tubular cells. In oliguric ATN, urine flow falls to about 20 mL/h, and BUN, serum creatinine, phosphate, and potassium levels rise. With nonoliguric AKI, the patient may remain in better fluid balance, but elimination of waste products is impaired. Ischemic injury to the kidneys can occur when the mean arterial blood pressure drops below 60 mm Hg for more than 30 minutes. Massive hemorrhage, transfusion reaction, sepsis, cardiovascular collapse, or major trauma can cause ischemic renal injury.
Substances that injure the kidneys are called nephrotoxins. The most common are medications, such as antibiotics and nonsteroidal antiinflammatory agents (NSAIDs). Other medications, including anesthetics and cancer chemotherapy agents, as well as street drugs, are toxic to the kidney in varying degrees. Radiologic contrast dye used for intravenous pyelography, cardiac catheterization, and computed tomography is potentially nephrotoxic. Other nephrotoxins include hemoglobin (from hemolysis of red blood cells) and myoglobin from muscle breakdown (rhabdomyolysis), caused by crush injury, heatstroke, or seizure.
Postrenal. Postrenal causes account for approximately 5% of AKI cases. Postrenal failure is usually the result of obstruction in the flow of urine anywhere from the kidney to the urinary meatus. The obstruction can be functional or mechanical. Functional causes include diabetic nephropathy, such medications as ganglionic blocking agents that block the autonomic nerve supply to the urinary system, and neurogenic bladder subsequent to spinal cord injury or cerebrovascular accident. Tumors, stones, prostatic hypertrophy, and urethral strictures are some mechanical causes of postrenal failure.
Box 18-1 Etiologies of Acute Kidney Injury
Prerenal (decreased renal perfusion)
Modified from Copstead LC, Banasik JL: Pathophysiology, ed 4, St. Louis, 2010, Mosby.
Does a patient with acute kidney injury have urine output?
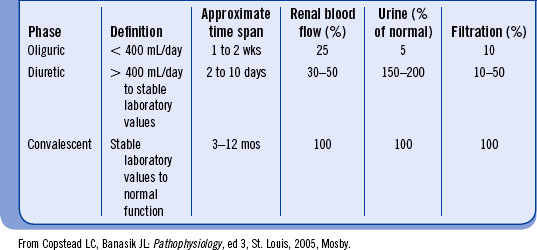
Some patients with AKI have significant urine output, referred to as nonoliguric renal failure. Most patients progress through several stages from oliguria to anuria to polyuria, depending on the phase of AKI (Table 18-2).
Oliguric-anuric phase. Oliguria is defined as urine volume less than 400 mL/day. Anuria is a urine output of less than 50 mL/day. This phase can last from 2 days to 30 days or longer. The longer the oliguria or anuria continues, the more the prospect of returning to normal urine output worsens. Proper management of fluid volume is essential.
Diuretic phase. This phase begins when the urine output reaches 1 L/day. The renal indices may stabilize and then start to approach normal with gradual return of renal function. A 24-hour urine volume can increase to as much as 4 to 5 L. Accurate evaluation of the patient’s status to avoid dehydration leading to hypoperfusion of the kidneys is mandatory. Laboratory values are monitored closely with the expectation that they will return to normal in the late phase of AKI.
Convalescent phase. This period begins with the stabilization of serum chemistries and gradual return of normal kidney function. This phase may last from three to six months. Return to normal glomerular filtration rate, if it occurs, may take up to a year.
What are the clinical presentations of acute kidney injury?
The clinical presentations of AKI include all of the symptoms, signs, and findings of rapidly developing uremia (see Chapter 5).
What biochemical changes are present in acute kidney injury?
The damaged kidneys are unable to excrete the products of normal body metabolism. There is elevation of the serum urea and creatinine, and altered electrolyte levels. Increased hydrogen ion concentration causes acidosis and a low serum pH. Hyperkalemia, hypokalemia, hypocalcemia, hyperphosphatemia, hypermagnesemia, and low bicarbonate may be observed.
What are the indications for treatment?
The most common indications for acute dialysis include the following:
Uremia. Acute dialysis is initiated when a patient becomes symptomatically uremic (see Chapter 5) regardless of BUN or creatinine level. Dialysis may be started prophylactically when the BUN reaches 100 mg/dL, even if the patient has few or no symptoms.
Pulmonary edema. Acute pulmonary edema is a life-threatening complication of AKI that necessitates immediate dialysis. Acute pulmonary edema can result from fluid overload directly attributable to AKI or as the result of an acute myocardial infarction or from overzealous administration of fluid.
Hyperkalemia. Hyperkalemia is a result of the damaged kidney’s inability to secrete potassium and the release of intracellular potassium (because of acidosis and tissue breakdown). Hemodialysis is effective in lowering potassium and is initiated when rapid reduction of plasma potassium is indicated. Peritoneal dialysis is an acceptable treatment option, although its effects are slower than that of hemodialysis. Hyperkalemia can be managed in an emergency, while waiting for hemodialysis, by intravenous administration of glucose and insulin in combination with intravenous sodium bicarbonate. These shift extracellular potassium into the cell, where it cannot cause cardiac arrhythmia. Calcium gluconate may be given intravenously to reduce myocardial irritability. Sodium polystyrene sulfonate cation exchange resin (Kayexalate) by mouth or by enema can be administered when slower correction of potassium is acceptable or in the initial management of hyperkalemia.
Acidosis. Metabolic acidosis is caused by the inability of the kidneys to excrete hydrogen ion and to reabsorb bicarbonate. Acidosis can be treated temporarily by intravenous sodium bicarbonate. Hemodialysis may be required because of the added sodium, which increases the danger of volume overload.
Neurologic changes. Toxic effects of uremia can result in central nervous system changes. Headache, insomnia, and drowsiness are early symptoms; confusion, convulsions, and coma may occur later. Dialysis is indicated when any of these serious symptoms are seen, and preferably before they occur.
Drug overdoses and poisonings. Dialysis is indicated for the treatment of some drug intoxications. Drugs normally excreted by the kidneys, or water-soluble drugs of low molecular weight, will diffuse rapidly across cellulosic dialysis membranes. Such drugs are readily removed with hemodialysis. Examples include ethanol, lithium, methanol, and salicylates. Water-soluble drugs with high molecular weight, such as vancomycin and amphotericin B, diffuse across cellulosic membranes much more slowly and are less well removed. If the intoxicant is protein bound (e.g., digoxin and acetylsalicylic acid) or lipid soluble (e.g., glutethimide), hemodialysis is not useful. However, both of these intoxicants are removed by hemoperfusion with a charcoal cartridge or with a plasma membrane filter.
What type of vascular access is used for acute dialysis?
The most common access to the circulation for acute dialysis is a double-lumen venous catheter. The catheter may be placed in the subclavian, internal jugular, or femoral vein. Insertion of a catheter into the subclavian or internal jugular vein must be followed by an x-ray examination to determine correct placement and to rule out pneumothorax or hemothorax before the catheter is used (see Chapter 12).
Can an arteriovenous fistula or graft be used for acute dialysis?
Patients with an arteriovenous (AV) fistula or graft may require acute dialysis, and the fistula or graft may be used after its patency is determined (see Chapter 12 for additional information).
What complications may occur with acute kidney injury?
Hypertension. Fluid removal by dialysis may correct the hypertension. Antihypertensive medication may be necessary.
Hypotension. Hypotension may result from blood loss, a strict fluid restriction, sepsis, myocardial infarction, or pericarditis. To prevent additional kidney damage, the underlying cause of the hypotension must be corrected to maintain adequate renal perfusion. The use of a vasopressor, such as dopamine, may be necessary.
Anemia. In AKI the release of erythropoietin is decreased. The usual response to therapy with recombinant erythropoietin or epoetin alfa requires three to four weeks, so it is not of immediate help. Uremic red blood cells also have a shortened life span. Blood loss from bleeding is often present (see Chapter 5 for additional information about complications).
Are there special precautions when dialyzing someone with acute kidney injury?
Patients requiring acute dialysis are generally critically ill with multisystem failure. A thorough and accurate total assessment of the patient is essential before initiating dialysis (see Chapter 13). The nephrology nurse must be highly cognizant of changes, be prudent in assessing and monitoring the patient’s vital signs, and respond appropriately.
Controlled anticoagulation with heparin may be necessary to minimize clotting of the dialyzer; however, dialysis can be performed with little or no heparin. Careful monitoring of clotting times may be necessary to prevent complications related to heparinization during hemodialysis. A baseline clotting time must be obtained before the start of dialysis; a normal activated clotting time (ACT) is 60 to 90 seconds. The Clinical Laboratory Improvement Act (CLIA) now requires quality control checks for ACT machines to meet CLIA requirements. These requirements are resource intensive and may not permit bedside monitoring of ACT levels during dialysis (see Chapter 11).
The physician determines the technique of anticoagulation. “Tight” or no heparin may be used for patients at high risk for bleeding. In tight heparinization, clotting times are performed every 30 minutes. The ACT is kept at 1.25 times the baseline with additional heparin, as indicated when the ACT falls below the 1.25 baseline value. With systemic heparinization, generally the ACT values are allowed to range from 2.5 to 3 times the baseline.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree