Acid-Base Compensation
QUICK LOOK AT THE CHAPTER AHEAD
Compensation is the body’s attempt to bring the acid-base imbalance back to normal. When compensating an acid-base disturbance, the system not responsible for the imbalance attempts to bring the blood pH back to normal. Compensation, however, is not correction. Unless the underlying condition is rectified, the imbalance is not corrected. In this chapter we discuss the compensatory mechanisms for acid-base disturbances.
As mentioned previously, the body has built-in mechanisms to compensate for acid-base imbalances. Compensation, however, is not correction. It is the body’s attempt to bring the imbalance back to normal. Unless the underlying condition is rectified, the imbalance is not corrected. When compensating an acid-base disturbance, the system not responsible for the imbalance attempts to bring the blood pH back to normal.
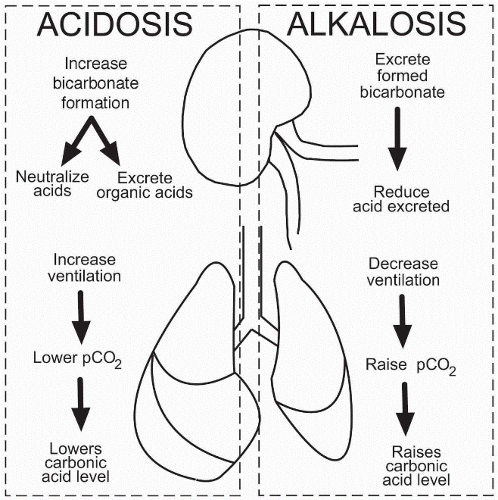
In metabolic acidosis the respiratory system attempts to compensate by increasing the rate and depth of respirations through hyperventilation. When the pH drops below normal, the peripheral chemoreceptors stimulate the respiratory system in the brainstem. This increases the rate and
depth of respirations in an attempt to blow off CO2 and therefore to lower the carbonic acid blood level. By lowering the carbonic acid level, there is less acid in the blood for the bicarbonate to buffer, helping to raise the bicarbonate levels. The arterial blood gases in a patient with a compensated metabolic acidosis show a slightly low or normal pH level of 7.35-7.40, a decreased HCO3 (initial problem) level less than 23 mEq/L, and a decreased PCO2 (compensatory response) less than 35 mm Hg.
depth of respirations in an attempt to blow off CO2 and therefore to lower the carbonic acid blood level. By lowering the carbonic acid level, there is less acid in the blood for the bicarbonate to buffer, helping to raise the bicarbonate levels. The arterial blood gases in a patient with a compensated metabolic acidosis show a slightly low or normal pH level of 7.35-7.40, a decreased HCO3 (initial problem) level less than 23 mEq/L, and a decreased PCO2 (compensatory response) less than 35 mm Hg.
 In metabolic acidosis the respiratory system attempts to compensate by increasing the rate and depth of respirations through hyperventilation. Blowing off CO2 eliminates excess acid from the body, allowing for more bicarbonate to buffer the remaining acid.
In metabolic acidosis the respiratory system attempts to compensate by increasing the rate and depth of respirations through hyperventilation. Blowing off CO2 eliminates excess acid from the body, allowing for more bicarbonate to buffer the remaining acid.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access