Acid-Base
QUICK LOOK AT THE CHAPTER AHEAD
In this chapter we discuss the hydrogen ion and its relationship to pH. The regulation of pH through the buffering systems is also identified.
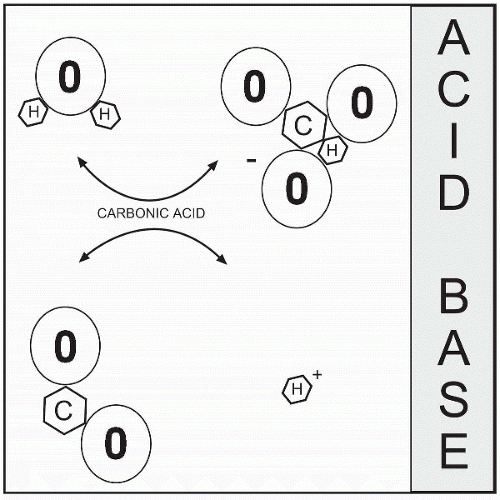
The amount of acid or base in body fluid is reflected in the pH, the negative logarithm of the hydrogen ion (H+). The hydrogen ion (acid) is needed for maintenance of cellular membranes and enzyme reactions, and minor alterations may affect metabolism and essential body functions. It travels in the body fluid as a volatile acid, carbonic acid (H2CO3). It breaks down into H+ and HCO3–. The volatile gas, CO2, is expelled through breathing, and the remaining part of the compound forms with other ions to make nonvolatile acids that are excreted in the urine. Nonvolatile acids such as the noncarbonic acids (hydrochloric, phosphoric, etc.) are buffered and then eliminated via the renal system, not the respiratory system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree