A
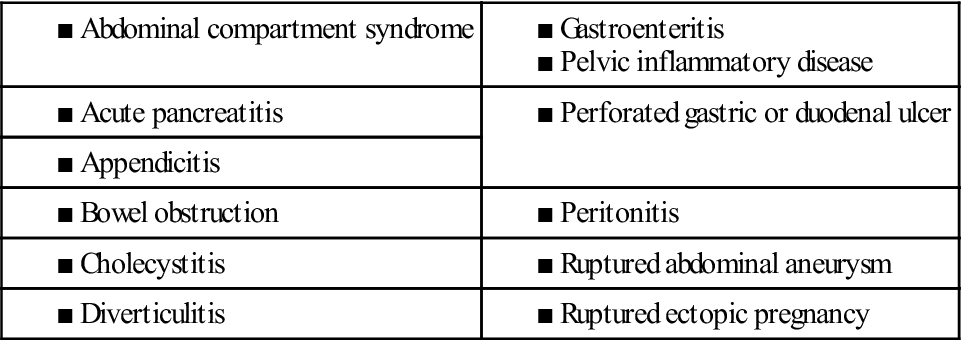
Abdominal pain, acute
Description
Acute abdominal pain is pain of recent onset. It may signal a life-threatening problem and therefore requires immediate attention. Causes include damage to organs in the abdomen and pelvis, which leads to inflammation, infection, obstruction, bleeding, and perforation. The most common causes of acute abdominal pain are listed in Table 1.
Clinical manifestations
Pain is the most common symptom of an acute abdominal problem. Patients may also complain of nausea, vomiting, diarrhea, constipation, flatulence, fatigue, fever, and bloating.
Diagnostic studies
Diagnosis begins with a complete history and physical examination. Description of the pain (frequency, timing, duration, location), accompanying symptoms, and sequence of symptoms (e.g., pain before or after vomiting) provide vital clues about the problem. Physical examination should include both a rectal and pelvic examination in addition to an abdominal examination.
Collaborative care
The goal of management is to identify and treat the cause and monitor and treat complications, especially shock. Table 43-10 in Lewis et al., Medical-Surgical Nursing, ed 9, p. 971, outlines emergency management of the patient with acute abdominal pain.
Nursing management
Goals
The patient will have resolution of inflammation, relief of abdominal pain, freedom from complications (especially hypovolemic shock), and normal nutritional status.
Nursing interventions
General care involves management of fluid and electrolyte imbalances, pain, and anxiety. Assess the quality and intensity of pain at regular intervals, and provide medication and other comfort measures. Maintain a calm environment and provide information to help allay anxiety. Conduct ongoing assessments of vital signs, intake and output, and level of consciousness, which are key indicators of hypovolemic shock.
Preoperative care includes the emergency care of the patient and general care of the preoperative patient (Chapter 18 and Table 43-10 Lewis et al., Medical-Surgical Nursing, ed 9, p. 971).
Postoperative care depends on the type of surgical procedure performed. Laparoscopic procedures result in lower rates of postoperative complications (e.g., poor wound healing, paralytic ileus), earlier diet advancement, and shorter hospital stays compared with open surgical procedures. A general nursing care plan (eNCP 20-1) for the postoperative patient is presented on the website for Chapter 20.
A nasogastric (NG) tube with low suction may be used to empty the stomach and prevent gastric dilation. If the upper gastrointestinal (GI) tract has been entered, drainage from the NG tube may be dark brown to dark red for the first 12 hours. Later it should be light yellowish brown or greenish. If a dark red color continues or if bright red blood is observed, notify the surgeon because of the possibility of hemorrhage. “Coffee ground” granules in the drainage indicate blood that has been modified by acidic gastric secretions.
■ Nausea and vomiting are common after a laparotomy and may be caused by the surgery, decreased peristalsis, or pain medication. Antiemetics such as prochlorperazine (Compazine), ondansetron (Zofran), or trimethobenzamide (Tigan) may be ordered (see Nausea and Vomiting, p. 432).
■ Monitor fluid and electrolyte status along with blood pressure, heart rate, and respirations.
 Patient and caregiver teaching
Patient and caregiver teaching
Preparation for discharge begins soon after surgery. Teach the patient and caregiver about any modifications in activity, care of the incision, diet, and drug therapy.
Acute coronary syndrome
Description
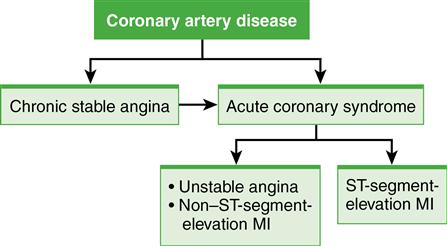
Acute coronary syndrome (ACS) develops when ischemia is prolonged and not immediately reversible. ACS encompasses the spectrum of unstable angina (UA), non–ST-segment-elevation myocardial infarction (NSTEMI), and ST-segment-elevation myocardial infarction (STEMI) (Fig. 1). Although each remains a distinct diagnosis, this nomenclature (ACS) reflects the relationships among the pathophysiology, presentation, diagnosis, prognosis, and interventions for these disorders.
Pathophysiology
ACS is associated with deterioration of a once-stable atherosclerotic plaque. The plaque then ruptures, exposing the intima to blood and stimulating platelet aggregation and vasoconstriction with thrombus formation. This unstable lesion may be partially occluded by a thrombus (manifesting as UA or NSTEMI) or totally occluded by a thrombus (manifesting as STEMI).
What causes a coronary plaque to suddenly become unstable is not well understood, but systemic inflammation is thought to play a role.
Unstable angina
The patient with chronic stable angina may develop UA, or UA may be the first clinical manifestation of CAD. Unlike chronic stable angina, UA is unpredictable and represents an emergency.
Myocardial infarction
A myocardial infarction (MI) occurs because of sustained ischemia, causing irreversible myocardial cell death (necrosis). Thrombus formation is responsible for 80% to 90% of all acute MIs. When a thrombus develops, perfusion to the myocardium distal to the occlusion is blocked, resulting in necrosis. Contractile function of the heart stops in the necrotic areas. The degree of altered function depends on the area of the heart involved and the size of the infarction. Most MIs involve some portion of the left ventricle.
Cardiac cells can withstand ischemic conditions for approximately 20 minutes before cellular death (necrosis) begins. If ischemia persists, it takes approximately 4 to 6 hours for the entire thickness of the heart muscle to become necrosed.
The body’s response to cell death is the inflammatory process. Within 24 hours, leukocytes infiltrate the area. Enzymes are released from the dead cardiac cells and are important diagnostic indicators of MI. Proteolytic enzymes from neutrophils and macrophages remove all necrotic tissue by the fourth day.
At 10 to 14 days after an MI, the new scar tissue is still weak. The myocardium is vulnerable to increased stress because of the unstable state of the healing heart wall. Changes in the infarcted muscle also alter the unaffected myocardium. In an attempt to compensate for the infarcted muscle, the normal myocardium hypertrophies and dilates. Remodeling of normal myocardium can lead to the development of late heart failure (HF).
Clinical manifestations
Unstable angina
The chest pain associated with UA is new in onset, occurs at rest, or has a worsening pattern. Women seek medical attention for symptoms of UA more often than men. Despite national efforts to increase awareness, women’s symptoms (fatigue, shortness of breath, indigestion, anxiety) continue to go unrecognized as related to heart problems.
Myocardial infarction
Severe, immobilizing, and persistent chest pain not relieved by rest or nitrate administration is the hallmark of an MI.
Additional manifestations may include nausea and vomiting, diaphoresis, and the patient’s skin may be ashen, clammy, and cool (cold sweat). Fever occurs within the first 24 hours (up to 100.4° F [38° C]) and may continue for 1 week. BP and pulse rate are also elevated initially. The BP may then drop, with decreased urine output, lung crackles, hepatic engorgement, and peripheral edema. Jugular veins may be distended with obvious pulsations.
Complications
■ Dysrhythmias are the most common complication after an MI and are the most common cause of death in patients in the prehospital period. Dysrhythmias are caused by any condition that affects the myocardial cell’s sensitivity to nerve impulses, such as ischemia, electrolyte imbalances, and sympathetic nervous system stimulation. The intrinsic rhythm of the heart is disrupted, causing either a very fast heart rate (HR) (tachycardia), a very slow HR (bradycardia), or irregular HR. Life-threatening dysrhythmias occur most often with anterior wall infarction, heart failure, and shock. Complete heart block is seen in a massive infarction (see Dysrhythmias, p. 202).
Diagnostic studies
In addition to the patient’s history of pain, risk factors, and health history, the primary diagnostic studies used to determine whether a person has UA or an MI include an ECG and serum cardiac markers. Other diagnostic measures can include coronary angiography, exercise stress testing, and echocardiogram.
ECG
Cardiac markers
Certain proteins, called serum cardiac markers, are released into the blood from necrotic heart muscle after an MI.
Collaborative care
It is extremely important that a patient with ACS is rapidly diagnosed and treated to preserve cardiac muscle. Initial management of the patient with chest pain most often occurs in the emergency department (ED). Emergency care of the patient with chest pain is presented in Table 34-12, Lewis et al, Medical-Surgical Nursing, ed 9, p. 750.
The goal in treatment of acute MI is to salvage as much myocardial muscle as possible.
■ Reperfusion therapy is indicated for patients with STEMI or NSTEMI with positive cardiac markers. Reperfusion therapy can include emergent PCI or fibrinolytic (thrombolytic) therapy. Emergent PCI is recommended as the first line of treatment for patients with confirmed MI. The patient undergoes cardiac catheterization to evaluate the blockage, and stents may be placed (see Angina, Chronic Stable, p. 46).
Thrombolytic therapy aims to stop the infarction process by dissolving the thrombus in the coronary artery and reperfusing the myocardium. Thrombolytic therapy (e.g., reteplase [Retavase]) is given as soon as possible, ideally within the first hour and preferably within the first 6 hours after the onset of symptoms. Mortality is reduced by 25% if reperfusion occurs within 6 hours. Contraindications and complications with thrombolytic therapy are described in Lewis et al, Medical-Surgical Nursing, ed 9, p. 751.
Coronary artery bypass graft (CABG) surgery consists of the placement of conduits to transport blood between the aorta, or other major arteries, and the myocardium distal to the obstructed coronary artery (or arteries). It requires a sternotomy (opening of the chest cavity) and the use of cardiopulmonary bypass (CPB). It is a palliative treatment for CAD and not a cure. Newer techniques include minimally invasive direct coronary artery bypass and transmyocardial laser revascularization. These surgical procedures and related nursing care are further discussed in Lewis et al, Medical-Surgical Nursing, ed 9, pp. 752 to 753.
Drug therapy includes IV nitroglycerin (Tridil), aspirin, β-adrenergic blockers, and systemic anticoagulation with either low-molecular-weight heparin given subcutaneously or IV unfractionated heparin as initial drug treatments of choice. Angiotensin-converting enzyme (ACE) inhibitors are added for select patients following MI, and calcium channel blockers may be used if the patient is already taking adequate doses of β-blockers or does not tolerate these blockers.
Nursing management
Goals
The patient with an MI will experience relief of pain, preservation of myocardium, immediate and appropriate treatment, effective coping with illness-associated anxiety, participation in a rehabilitation plan, and reduction of risk factors.
See NCP 34-1 for the patient with acute coronary syndrome, Lewis et al, Medical-Surgical Nursing, ed 9, pp. 755 to 756.
Nursing diagnoses
Nursing interventions
Priorities for nursing interventions in the initial phase include pain assessment and relief, physiologic monitoring, promotion of rest and comfort, alleviation of stress and anxiety, and understanding of the patient’s emotional and behavioral reactions. Proper management of these priorities decreases the O2 needs of a compromised myocardium. In addition, you should institute measures to avoid the hazards of immobility while encouraging rest.
■ Provide nitroglycerin, morphine, and O2 as needed to eliminate or reduce chest pain.
■ Assess the patient’s oxygenation status, especially if the patient is receiving O2. In addition, check the nares for irritation or dryness (see Oxygen Therapy, p. 717).
 Patient and caregiver teaching
Patient and caregiver teaching
Patient teaching needs to occur at every stage of the patient’s hospitalization and recovery (e.g., ED, telemetry unit, home care). The purpose of teaching is to give the patient and caregiver the tools they need to make informed health decisions (Table 2).
Table 2
Patient and Caregiver Teaching Guide
Acute Coronary Syndrome
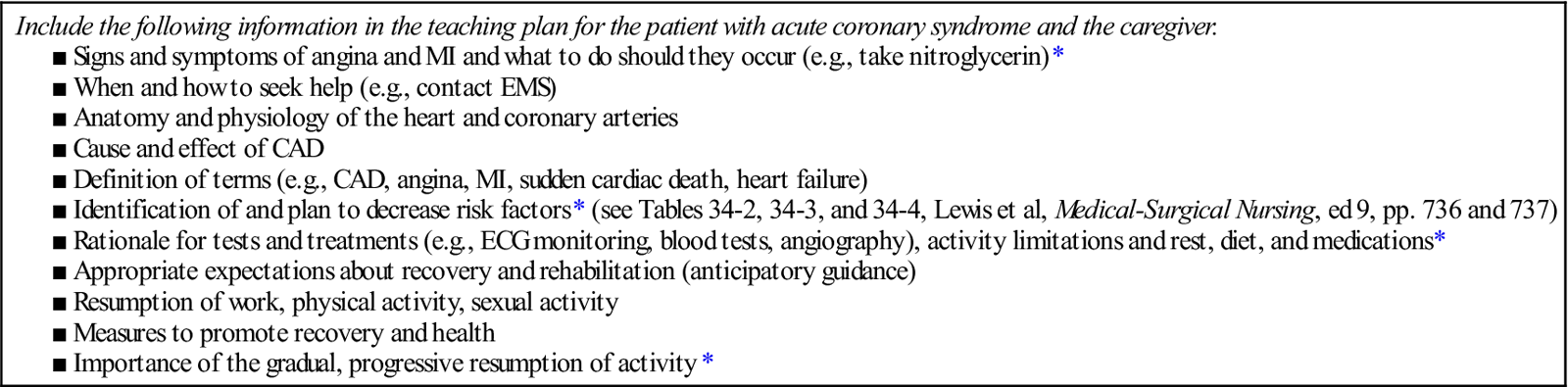
*Identified by patients as most important to learn before discharge.
Acute respiratory distress syndrome
Description
Acute respiratory distress syndrome (ARDS) is a sudden and progressive form of acute respiratory failure in which the alveolar-capillary membrane becomes damaged and more permeable to intravascular fluid. The alveoli fill with fluid, resulting in severe dyspnea, hypoxemia refractory to supplemental oxygen (O2), reduced lung compliance, and diffuse pulmonary infiltrates.
Incidence of ARDS in the United States is estimated at more than 150,000 cases annually. Despite supportive therapy, mortality from ARDS is approximately 50%. Patients who have both gram-negative septic shock and ARDS have a mortality rate of 70% to 90%.
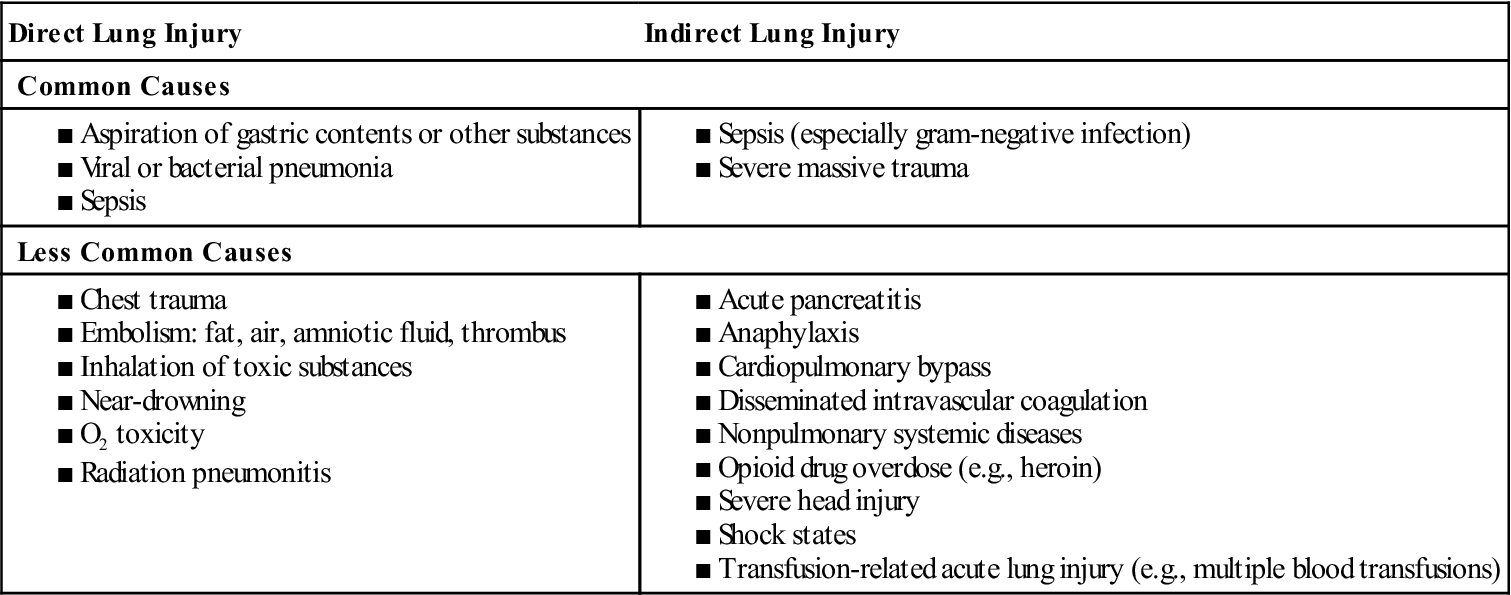
■ Table 3 lists conditions that predispose patients to the development of ARDS. The most common cause is sepsis. Patients with multiple risk factors are three or four times more likely to develop ARDS.
■ Direct lung injury may cause ARDS, or ARDS may develop as a consequence of the systemic inflammatory response syndrome (SIRS). ARDS may also develop as a result of multiple organ dysfunction syndrome (MODS) (see Systemic Inflammatory Response Syndrome and Multiple Organ Dysfunction Syndrome, p. 614).
Table 3
Conditions Predisposing Patients to Acute Respiratory Distress Syndrome
Pathophysiology
An exact cause for damage to the alveolar-capillary membrane is not known. However, many changes are thought to be caused by stimulation of the inflammatory and immune systems, which causes an attraction of neutrophils to the pulmonary interstitium. The neutrophils cause a release of biochemical, humoral, and cellular mediators that produce changes in the lung, including increased pulmonary capillary membrane permeability, destruction of elastin and collagen, formation of pulmonary microemboli, and pulmonary artery vasoconstriction. Pathophysiologic changes in ARDS are divided into three phases: injury or exudative, reparative or proliferative, and fibrotic.
The injury or exudative phase occurs approximately 1 to 7 days (usually 24 to 48 hours) after the initial direct lung injury or host insult. The primary changes of this phase are interstitial and alveolar edema (noncardiogenic pulmonary edema) and atelectasis.
The reparative or proliferative phase begins 1 to 2 weeks after the initial lung injury. During this phase there is an influx of granulocytes, monocytes, and lymphocytes and fibroblast proliferation.
■ If this phase persists, widespread fibrosis results. If this phase is stopped, the lesions resolve.
The fibrotic phase occurs approximately 2 to 3 weeks after the initial lung injury. This phase is also called the chronic or late phase of ARDS. By this time the lung is completely remodeled by collagenous and fibrous tissues. Diffuse scarring and fibrosis result in decreased lung compliance and decreased surface area for gas exchange. Pulmonary hypertension results from fibrosis.
Progression of ARDS varies among patients. Some patients survive the acute phase of lung injury, pulmonary edema resolves, and complete recovery occurs in a few days. Others go on to the fibrotic (late or chronic) phase requiring long-term mechanical ventilation, with a poor chance of survival.
Clinical manifestations
At the time of initial injury and for several hours to 1 to 2 days afterward, the patient may not exhibit respiratory symptoms.
As ARDS progresses, symptoms worsen because of increased fluid accumulation in the lungs and decreased lung compliance. Tachycardia, diaphoresis, changes in sensorium with decreased mentation, cyanosis, and pallor may be present. Chest auscultation usually reveals scattered to diffuse crackles and rhonchi.
Complications may develop as a result of ARDS itself or its treatment. The major cause of death in ARDS is MODS, often accompanied by sepsis. The vital organs most commonly involved are the kidneys, liver, and heart. The organ systems most often involved are the central nervous system (CNS) and hematologic and gastrointestinal systems.
Diagnostic studies
No precise criteria define ARDS. Findings that support a diagnosis of ARDS are the patient presents with refractory hypoxemia, a chest x-ray with new bilateral interstitial or alveolar infiltrates, and a pulmonary artery wedge pressure of 18 mm Hg or less with no evidence of heart failure.
Nursing and collaborative management
Goals
With appropriate therapy, the overall goals for the patient with ARDS include a partial pressure of oxygen in arterial blood (PaO2) of at least 60 mm Hg and adequate lung volume to maintain normal pH. A patient recovering from ARDS will experience a PaO2 within limits of normal for age or baseline values on room air, oxygen saturation in arterial blood (SaO2) greater than 90%, a patent airway, and clear lungs on auscultation.
Nursing diagnoses
Nursing diagnoses for the patient with ARDS may include, but are not limited to, those described under Respiratory Failure, Acute (p. 534).
Collaborative care
The collaborative care for acute respiratory failure is applicable to ARDS (see Respiratory Failure, Acute, pp. 538 to 541). Patients with ARDS are commonly cared for in critical care units.
O2 administration.
The goal of O2 therapy is to correct hypoxemia (see Oxygen Therapy, p. 717). Initially use masks with high-flow systems that deliver higher O2 concentrations to maximize O2 delivery. The general standard for O2 administration is to give the lowest concentration that results in a PaO2 of 60 mm Hg or greater. When FIO2 exceeds 60% for more than 48 hours, the risk for O2 toxicity increases. Patients with ARDS need intubation with mechanical ventilation (see Artificial Airways: Endotracheal Tubes, p. 679) because the PaO2 cannot otherwise be maintained at acceptable levels.
Mechanical ventilation.
Endotracheal intubation and positive pressure ventilation provide additional respiratory support. In patients with ARDS, positive expiratory-end pressure (PEEP) is often used. When PEEP is applied, the lung is kept partially expanded, which prevents the alveoli from totally collapsing. If hypoxemic failure persists in spite of high levels of PEEP, alternative modes and therapies may be used. These include airway pressure release ventilation, pressure-control inverse ratio ventilation, high-frequency ventilation, and permissive hypercapnia (low tidal volumes that allow PaCO2 to increase slowly).
Extracorporeal membrane oxygenation (ECMO) and extracorporeal carbon dioxide (CO2) removal pass blood across a gas-exchanging membrane outside the body and then return oxygenated blood back to the body.
Positioning.
Some patients with ARDS have a marked improvement in PaO2 when turned from the supine to the prone position (e.g., PaO2 70 mm Hg supine, PaO2 90 mm Hg prone) with no change in inspired O2 concentration. The response may be sufficient to allow a reduction in inspired O2 concentration or PEEP.
Another positioning strategy that you can consider for patients with ARDS is continuous lateral rotation therapy, which provides continuous, slow, side-to-side turning of the patient by rotating the actual bed frame. You should maintain the lateral movement of the bed for 18 of every 24 hours to stimulate postural drainage and help to mobilize pulmonary secretions. In addition, the bed may also contain a vibrator pack that can provide chest physiotherapy to assist with secretion mobilization and removal.
Medical supportive therapy
Patients on PPV and PEEP frequently experience decreased cardiac output. Hemodynamic monitoring is essential to see trends, detect changes, and adjust therapy as needed. An arterial catheter is inserted for continuous monitoring of blood pressure (BP) and sampling of blood for ABGs. Use of inotropic drugs, such as dobutamine (Dobutrex) or dopamine (Intropin), may also be necessary. Packed red blood cells are used to increase hemoglobin and thus the O2-carrying capacity of the blood.
Maintenance of nutrition and fluid balance is challenging in the patient with ARDS. Parenteral or enteral feedings are started to meet the high energy requirements of these patients. Increasing pulmonary capillary permeability results in fluid in the lungs and causes pulmonary edema. At the same time, the patient may be volume depleted and therefore prone to hypotension and decreased cardiac output from mechanical ventilation and PEEP. Controversy exists as to the benefits of fluid replacement with crystalloids versus colloids. The patient is usually placed on fluid restriction, and diuretics are used as necessary.
 Patient and caregiver teaching
Patient and caregiver teaching
Fear of suffocation or death is not uncommon in patients with ARDS.
Addison’s disease
Description
Addison’s disease is a primary adrenocortical insufficiency in which all three classes of adrenal steroids (glucocorticoids, mineralocorticoids, and androgens) are reduced because of hypofunction of the adrenal cortex.
In secondary adrenocortical insufficiency, which is caused by a lack of pituitary adrenocorticotropic hormone (ACTH) secretion, corticosteroids and androgens are deficient but mineralocorticoids rarely are.
The most common cause of Addison’s disease in the United States is an autoimmune response in which adrenal tissue is destroyed by antibodies against the patient’s own adrenal cortex. Often other endocrine conditions are present and Addison’s disease is considered a component of autoimmune polyglandular syndrome. Other causes include infarction, fungal infections (e.g., histoplasmosis), acquired immunodeficiency syndrome (AIDS), and metastatic cancer.
Addison’s disease may be caused by adrenal hemorrhage (often related to anticoagulant therapy), antineoplastic chemotherapy, ketoconazole therapy for AIDS, or bilateral adrenalectomy.
Clinical manifestations
Manifestations have a slow (insidious) onset and include progressive weakness, fatigue, weight loss, and anorexia. Skin hyperpigmentation, a striking feature due to increased ACTH, is seen primarily in sun-exposed areas of the body, at pressure points, over joints, and in creases, especially palmar creases.
Patients with adrenocortical insufficiency are at risk for acute adrenal insufficiency (addisonian crisis), which is a life-threatening emergency caused by insufficient adrenocortical hormones or a sudden, sharp decrease in these hormones.
Diagnostic studies
Collaborative care
Treatment is focused on management of the underlying cause when possible. The mainstay of treatment is hormone therapy with corticosteroids (e.g., hydrocortisone) with glucocorticoid and mineralocorticoid activity. Mineralocorticoid replacement with fludrocortisone acetate (Florinef) is administered daily with increased salt in the diet.
The patient in addisonian crisis requires immediate aggressive management. Treatment must be directed toward shock management and high-dose hydrocortisone replacement. Large volumes of 0.9% saline solution and 5% dextrose are administered to reverse hypotension and electrolyte imbalances until BP returns to normal.
Nursing management
When the patient with Addison’s disease is hospitalized, nursing management focuses on monitoring the patient while correcting fluid and electrolyte balance.
 Patient and caregiver teaching
Patient and caregiver teaching
The serious nature of the disease and the need for lifelong hormone therapy necessitate a comprehensive teaching plan (Table 4).
Table 4
Patient and Caregiver Teaching Guide
Addison’s Disease

Alzheimer’s disease
Description
Alzheimer’s disease (AD) is a chronic, progressive, degenerative disease of the brain. It is the most common form of dementia, accounting for about 60% to 80% of all cases of dementia (see Dementia, p. 171). Approximately 5.2 million people in the United States have AD. Ultimately the disease is fatal, with death typically occurring 4 to 8 years after diagnosis, although some patients live for 20 years. AD is the sixth leading cause of death in the United States.
Pathophysiology
The exact etiology of AD is unknown. AD is not a normal part of aging. When AD develops in someone younger than 60 years, it is referred to as early-onset AD. AD that becomes evident in individuals after the age of 60 years is called late-onset AD.
Characteristic findings in AD relate to changes in the brain’s structure and function, including amyloid plaques, neurofibrillary tangles, loss of connections between neurons, and neuron death.
Plaques and neurofibrillary tangles are not unique to patients with AD or dementia. They are also found in the brains of individuals without evidence of cognitive impairment. However, they are more abundant in the brains of individuals with AD.
The other features of AD are the loss of connections between neurons and neuron death. These processes result in structural damage. Affected parts of the brain begin to shrink in a process called brain atrophy. By the final stage of AD, brain tissue has shrunk significantly.
Genetic factors may play a critical role in the way that the brain processes the β-amyloid protein. Overproduction of β-amyloid appears to be an important risk factor for AD. Abnormally high levels of β-amyloid cause cell damage either directly or through eliciting an inflammatory response and ultimately neuron death.
Diabetes mellitus, hypertension, current smoking, hypercholesterolemia, obesity, and trauma are associated with an increased risk of dementia including AD.
Clinical manifestations
Pathologic changes often precede clinical manifestations of dementia by 5 to 20 years. (Early warning signs of AD are listed in Table 5.) The rate of progression from mild to severe is highly variable and ranges from 3 to 20 years.
Table 5
Patient and Caregiver Teaching Guide
Early Warning Signs of Alzheimer’s Disease
| Include the following information in the teaching plan for the patient with Alzheimer’s disease and the family caregiver. | |
| Warning Sign | Description |
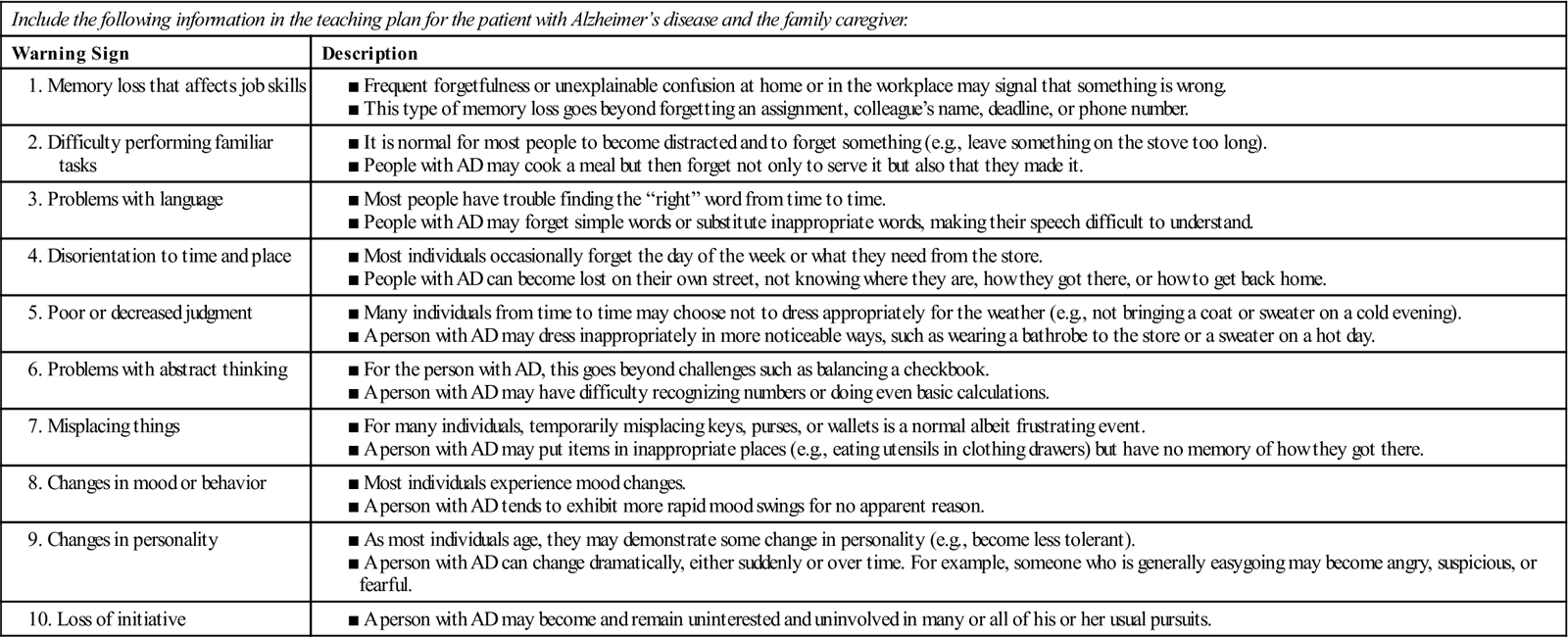
Adapted from Alzheimer’s Association: Early warning signs, Chicago, The Association.
Initial manifestations are usually related to changes in cognitive functioning. Patients may have complaints of memory loss, mild disorientation, and/or trouble with words and numbers. Often it is a family member, in particular the spouse, who reports the patient’s declining memory to the health care provider.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree