Patients transitioning from IV therapy to subcutaneous administration should administer the first subcutaneous dose instead of the next scheduled IV dose. Abatacept is dosed weekly in the subcutaneous regimen and may be initiated with or without an IV loading dose. If the subcutaneous regimen is initiated with an IV loading dose, determine loading dose as outlined in the previous chart. The first subcutaneous injection should be administered within a day of the IV loading dose.
Pediatric dose
Pediatric patients 6 to 17 years of age who weigh less than 75 kg:
10 mg/kg/dose based on patient’s body weight at each administration. After the initial dose, repeat administration at 2 and 4 weeks. Administer every 4 weeks thereafter.
Pediatric patients 6 to 17 years of age who weigh more than 75 kg:
See Usual Dose and the Abatacept Adult Dosing Guidelines chart. Do not exceed a maximum dose of 1,000 mg.
Dose adjustments
There is a trend toward a higher clearance with increasing body weight; see Usual Dose. No specific dose adjustments are required based on age or gender when corrected for body weight. ■ Withhold therapy in patients with severe infections. ■ The effects of renal or hepatic impairment have not been studied.
Dilution
Using ONLY the silicone-free disposable syringe provided with each vial and an 18- to 21-gauge needle, reconstitute each 250-mg vial with 10 mL SWFI; final concentration is 25 mg/mL. (If reconstituted with a siliconized syringe, the solution must be discarded.) Direct stream of SWFI toward side of vial. Do not use vial if vacuum is not present. Rotate or swirl vial gently until contents have dissolved. Do not shake. After dissolution, vent vial with a needle to dissipate any foam that may be present. Solution should be clear and colorless to pale yellow. Reconstituted solution must be further diluted to 100 mL as follows: From a 100-mL infusion bag or bottle, withdraw a volume of NS equal to the volume of reconstituted abatacept solution required for the patient’s dose (for 2 vials, remove 20 mL; for 3 vials, remove 30 mL; for 4 vials, remove 40 mL). Using the same silicone-free disposable syringe provided, slowly add the reconstituted abatacept into the infusion bag or bottle. Mix gently.
Filter:
Administration through a 0.2- to 1.2-micron, nonpyrogenic, low–protein binding filter is required.
Storage:
Refrigerate unopened vials at 2° to 8° C (36° to 46° F). Do not use beyond expiration date. Protect from light. Before administration, the diluted solution may be stored at RT or refrigerated; however, infusion of the diluted solution should be completed within 24 hours of reconstitution. Discard diluted solution if not administered within 24 hours. Any unused portion in a vial must be discarded.
Compatibility
Manufacturer states, “Should not be infused concomitantly in the same intravenous line with other agents.” Compatibility studies have not been performed.
Rate of administration
Administration through a 0.2- to 1.2-micron, nonpyrogenic, low–protein binding filter is required.
A single dose equally distributed over 30 minutes.
Actions
A soluble fusion protein that consists of the extracellular domain of human cytotoxic, T-lymphocyte–associated antigen 4 linked to the modified Fc portion of human immunoglobulin G1 (IgG1). Produced by recombinant DNA technology. Acts as a selective biologic response modulator by inhibiting T-lymphocyte activation, which is implicated in the pathogenesis of rheumatoid arthritis. Reduces pain and joint inflammation and slows the progression of structural damage to bone and cartilage. Mean half-life is 13.1 days (range 8 to 25 days).
Indications and uses
Reduce the S/S, induce a major clinical response, inhibit the progression of structural damage, and improve the physical function in adult patients with moderately to severely active rheumatoid arthritis. May be used as monotherapy or concomitantly with DMARDs other than TNF antagonists. SC injection may be used in adult patients unable to receive an infusion, and/or adult infusion patients may transition to SC injection; see prescribing information. ■ Reduce the S/S in pediatric patients 6 years of age and older with moderately to severely active polyarticular juvenile idiopathic arthritis. May be used as monotherapy or concomitantly with methotrexate.
Limitations of use:
Should not be administered concomitantly with TNF antagonists (e.g., adalimumab [Humira], etanercept [Enbrel]) or other biologic rheumatoid arthritis therapy, such as anakinra (Kineret).
Contraindications
Known hypersensitivity to abatacept or any of its components (maltose, monobasic sodium phosphate). See Limitations of Use and Drug/Lab Interactions.
Precautions
Concurrent use with a TNF antagonist (e.g., adalimumab [Humira], etanercept [Enbrel]) is associated with an increased risk of infections with no associated increased efficacy when compared with use of the TNF antagonist alone. Concurrent use is not recommended; see Contraindications and Drug/Lab Interactions. ■ Hypersensitivity reactions, including anaphylaxis, have been reported and can occur after the first infusion. Emergency medical equipment and medications for treating these reactions must be readily available. ■ Serious infections, including sepsis and pneumonia, have been reported; some have been fatal. Patients receiving concomitant immunosuppressive therapy may be at increased risk. ■ Use caution in patients with a history of recurrent infections, underlying conditions that may predispose them to infections, or chronic, latent, or localized infections; see Monitor. ■ Antirheumatic therapies have been associated with hepatitis B reactivation. Screening for viral hepatitis should be done before starting therapy with abatacept. Patients who screened positive for hepatitis were excluded from clinical studies. ■ Use with caution in patients with COPD. May be at increased risk for developing respiratory adverse events (e.g., COPD exacerbation, cough, dyspnea, rhonchi). ■ A small number of patients have developed binding antibodies to abatacept. No correlation of antibody development to clinical response or adverse events has been observed. ■ T-cells mediate cellular immune responses. Therefore drugs that inhibit T-cell activation, including abatacept, may affect patient defenses against infection and malignancies. The impact of abatacept on the development and course of malignancies is not fully understood. ■ See Maternal/Child.
Monitor:
Evaluate patients for latent tuberculosis (TB) with a TB skin test. Patients testing positive in TB screening should be treated with a standard TB regimen before initiating therapy with abatacept. ■ Screening for viral hepatitis is recommended before initiating therapy with abatacept; virus may reactivate with abatacept treatment. ■ Monitor for S/S of infection, especially if transitioning patient from TNF antagonist therapy to therapy with abatacept. Discontinue therapy if a serious infection develops. ■ Monitor COPD patients for worsening of respiratory status. ■ Monitor for S/S of hypersensitivity or infusion-related reactions; see Side Effects. ■ Do not administer live virus vaccines during or within 3 months of use. ■ See Precautions and Drug/Lab Interactions.
Patient education:
Read manufacturer’s patient information sheet before each infusion. ■ Review medication list and vaccination status with physician; see Precautions. ■ Report S/S of allergic reaction (e.g., rash, itching, wheezing), infusion reaction (e.g., dizziness, headache), or infection promptly. Discuss previous infections, current infections, or exposure to TB.
Maternal/child:
Category C: safety for use in pregnancy has not been established. Has been shown to cross the placenta in animal studies. Use caution. ■ Discontinue breast-feeding. ■ A pregnancy registry has been established; contact manufacturer. ■ Safety and effectiveness for use in pediatric patients under 6 years of age not established. ■ Safety and effectiveness for uses other than juvenile idiopathic arthritis in pediatric patients have not been established. ■ Safety and effectiveness of subcutaneous injection is not known in patients under 18 years of age. ■ Patients with juvenile idiopathic arthritis should be brought up-to-date with all immunizations before initiating therapy with abatacept.
Elderly:
Specific differences in safety and efficacy not noted. Incidence of infection and malignancy is higher in the elderly. Use caution; see Precautions.
Drug/lab interactions
Formal drug interaction studies have not been conducted. ■ Has been used with methotrexate, NSAIDs (e.g., naproxen [Naprosyn, Aleve], ibuprofen [Motrin, Advil]), corticosteroids (e.g., prednisone), azathioprine, chloroquine (Aralen), gold (Myochrysine), hydroxychloroquine (Plaquenil), leflunomide (Arava), and sulfasalazine (Azulfidine). ■ Methotrexate, NSAIDs, corticosteroids, and TNF antagonists do not appear to influence abatacept clearance. ■ Concurrent use with a TNF antagonist (e.g., adalimumab [Humira], etanercept [Enbrel], infliximab [Remicade]) is associated with an increased risk of serious infections and no significant additional efficacy over use of the TNF antagonist alone. Concurrent use is not recommended. ■ With the IV formulation, falsely elevated blood glucose readings may occur on the day of the infusion with specific blood glucose monitoring systems that react to drug products containing maltose. IV formulation contains maltose; SC formulation does not contain maltose; see prescribing information. ■ Safety and efficacy of concurrent use with anakinra (Kineret) has not been established. Concurrent use is not recommended. ■ Live virus vaccines should not be given concurrently with or within 3 months of abatacept. ■ May blunt the effectiveness of some vaccinations.
Side effects
In adult and pediatric patients, side effects are similar in type and frequency. The most commonly reported side effects are headache, nasopharyngitis, nausea, and upper respiratory tract infections. The most serious adverse effects are infections and malignancies. Infections are the most likely adverse event to cause interruption or discontinuation of therapy. Acute infusion-related reactions (cough, dyspnea, dizziness, flushing, headache, hypertension, hypotension, nausea, pruritus, rash, urticaria, wheezing) have been reported and usually occur within 1 hour of the infusion. Hypersensitivity reactions (anaphylaxis [rare], dyspnea, hypotension, urticaria) have been reported, usually within 24 hours of infusion. Other reactions include back or extremity pain, COPD exacerbation, dyspepsia, immunogenicity (antibody formation), and rhonchi.
Post-marketing:
Vasculitis (including cutaneous vasculitis and leukocytoclastic vasculitis).
Antidote
Notify physician of any side effects; most will be treated symptomatically. During clinical studies, most infusion-related reactions were mild to moderate, and therapy was discontinued in very few patients. Discontinue abatacept for any serious reaction or infection. Therapy may need to be interrupted in patients who develop infections. Treat infusion and hypersensitivity reactions as indicated (e.g., oxygen, diphenhydramine, epinephrine, corticosteroids, vasopressors, and/or fluids). Resuscitate as necessary.
Abciximab
(ab-SIX-ih-mab)
ReoPro
Antiplatelet agent
Antithrombotic
Monoclonal antibody
pH 7.2
Usual dose
Administered concomitantly with heparin and aspirin as described in Clinical Studies; see prescribing information.
Percutaneous coronary intervention:
0.25 mg/kg as an IV bolus administered 10 to 60 minutes before percutaneous coronary intervention (PCI). Follow with a continuous infusion of 0.125 mcg/kg/min (weight adjusted) to a maximum of 10 mcg/min (non–weight adjusted) for 12 hours.
Unstable angina not responding to conventional medical therapy with planned PCI within 24 hours:
0.25 mg/kg as an IV bolus followed by an 18- to 24-hour continuous infusion of 10 mcg/min. Discontinue abciximab 1 hour after the PCI.
Based on an integrated analysis of data from all studies, the following guidelines may be used to minimize the risk for bleeding:
• When abciximab is initiated 18 to 24 hours before PCI, the aPPT should be maintained between 60 and 85 seconds during the abciximab and heparin infusion period.
• During PCI, the ACT should be maintained between 200 and 300 seconds.
• If anticoagulation is continued in these patients following PCI, the aPTT should be maintained between 55 and 75 seconds.
Dilution
Available in 5-mL vials (2 mg/mL). Solution must be clear. Must be filtered with a nonpyrogenic, low–protein binding, 0.2- or 5-micron filter before administering the bolus and a 0.2- or 0.22-micron filter before administering the infusion; see Filters. Filtering of the infusion may be done during preparation or at administration, using the appropriate in-line filter. Do not shake.
IV injection:
Bolus injection may be given undiluted.
Infusion:
Withdraw desired dose and further dilute with NS or D5W (5 mL [10 mg] diluted with 250 mL NS or D5W equals 40 mcg/mL).
Filters:
Must be filtered before administering the bolus and the infusion. Bolus may be given using a sterile, nonpyrogenic, low–protein binding, 0.2- or 5-micron syringe filter. Filtering of the infusion may be done during preparation using a sterile, nonpyrogenic, low–protein binding, 0.2- or 5-micron syringe filter or at administration using an in-line, sterile, nonpyrogenic, low–protein binding, 0.2- or 0.22-micron filter; see Dilution.
Storage:
Refrigerate before use. Do not freeze. Check expiration date on vial. Contains no preservative; discard any unused portion.
Compatibility
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
According to the manufacturer, no incompatibilities have been shown with IV fluids or commonly used cardiovascular drugs; however, administration through a separate IV line and not mixing with other medications is recommended. No incompatibilities observed with glass bottles or polyvinyl chloride bags and administration sets.
One source suggests the following compatibilities:
Y-site:
Adenosine (Adenocard), argatroban, atropine, bivalirudin (Angiomax), diphenhydramine (Benadryl), fentanyl, metoprolol (Lopressor), midazolam (Versed).
Rate of administration
IV injection:
An initial dose as a bolus injection; filtration required.
Infusion:
See Usual Dose. Must be administered through an in-line, nonpyrogenic, low–protein binding filter (0.2 or 0.22 microns), if not done during preparation, and controlled by a continuous infusion pump. A 40-mcg/mL solution (10 mg in 250 mL) at a rate of 10.5 mL/hr will deliver 7 mcg/min, and 15 mL/hr will deliver 10 mcg/min. Discard unused portion at the end of the infusion.
Actions
The fab fragment of the chimeric human-murine monoclonal antibody, abciximab binds to the glycoprotein GPIIb/IIIa receptor of human platelets and produces rapid dose-dependent inhibition of platelet function. It inhibits platelet aggregation by preventing the binding of fibrinogen, von Willebrand factor, and other adhesive molecules to GPIIb/IIIa receptor sites on activated platelets. Also binds to the vitronectin receptor found on platelets and on the endothelial and smooth muscle cells of the vessel wall. The vitronectin receptor mediates the procoagulant properties of platelets and the proliferative properties of vascular endothelial and smooth muscle cells. Onset of action is rapid, reducing platelet aggregation to less than 20% of baseline within 10 minutes. Inhibition of platelet function is temporary following a bolus dose, but can be sustained at greater than 80% by continuous IV infusion. Has prevented acute thrombosis and resulted in lower rates of thrombosis as compared to aspirin and/or heparin. Initial half-life is 10 minutes. Second phase half-life is 30 minutes. After the infusion is ended, platelet function generally recovers gradually over 48 hours. In most patients, bleeding time returns to less than 12 minutes within 12 to 24 hours. Some abciximab remains in the circulation for 15 days or more.
Indications and uses
An adjunct to PCI for the prevention of cardiac ischemic complications in patients undergoing PCI and in patients with unstable angina not responding to conventional medical therapy when PCI is planned within 24 hours. Safety and effectiveness of abciximab use in patients not undergoing PCI have not been established. Used concurrently with aspirin and heparin.
Contraindications
Active internal bleeding, administration of oral anticoagulants (e.g., warfarin [Coumadin]) within 7 days unless PT is at or less than 1.2 times control, aneurysm, arteriovenous malformation, bleeding diathesis, clinically significant GI or GU bleeding within 6 weeks, history of CVA within 2 years, history of CVA with significant residual neurologic deficit, history of vasculitis (presumed or documented), hypertension (severe and uncontrolled), intracranial neoplasm, known hypersensitivity to any component of abciximab or to murine proteins, major surgery or trauma within 6 weeks, thrombocytopenia (less than 100,000/mm3), or the use of IV dextran before PCI or intent to use it during PCI.
Precautions
Administered only in the hospital under the direction of a physician knowledgeable in its use and with appropriate diagnostic, laboratory, and surgical facilities available. ■ May cause major bleeding complications (e.g., retroperitoneal bleeding, spontaneous GI and GU bleeding, bleeding at the arterial access site). Fatalities have occurred. ■ Risk of bleeding may be minimized by using weight-adjusted dosing of abciximab and low-dose weight-adjusted doses of heparin, with adherence to stricter anticoagulation guidelines, careful vascular access site management, discontinuation of heparin after the procedure, and early sheath removal. ■ Incidence of major bleeding is increased in patients receiving heparin, other anticoagulants, or thrombolytics (e.g., alteplase [tPA], reteplase [r-PA], streptokinase). Consider if benefits will outweigh risks, and proceed with extreme caution if use is considered necessary. ■ Incidence of major bleeding is also increased if PCI occurs within 12 hours of the onset of symptoms of an acute MI, if the PCI procedure is prolonged (lasting more than 70 minutes), or if PCI procedure fails. ■ Extreme care must be taken in accessing the femoral artery for femoral sheath placement. Only the anterior wall of the femoral artery should be punctured (avoid a Seldinger [through and through technique] for obtaining sheath access). ■ Avoid femoral vein sheath placement if possible. ■ Hypersensitivity reactions, including anaphylaxis, can occur at any time (a protein solution). Emergency drugs and equipment must always be available. ■ Thrombocytopenia, including severe thrombocytopenia, has been reported. Usually seen within the first 24 hours of abciximab administration. ■ Administration may result in the formation of human antichimeric antibody (HACA). Can cause hypersensitivity reactions including anaphylaxis, thrombocytopenia, or diminished benefit if abciximab is readministered at another time or other monoclonal antibodies are administered. Incidence and severity of thrombocytopenia may be increased with readministration. ■ See Drug/Lab Interactions.
Monitor:
Before initiating, obtain results of baseline CBC, platelet count, PT, ACT, and aPTT. Type and cross-match would also be appropriate. ■ Monitor heparin anticoagulation (ACT or aPTT) and PT closely. ■ While a femoral sheath is in place, the patient must be on strict bed rest, head of the bed should be less than 30 degrees, and the appropriate limb(s) restrained in a straight position. Monitor sheath insertion site(s) and distal pulses of affected leg(s) frequently while sheath is in place and for 6 hours after removal. Measure any hematoma and monitor for enlargement. ■ Monitor platelet count 2 to 4 hours following the bolus dose and at 24 hours or before discharge, whichever is first. More frequent monitoring may be indicated. ■ Monitor patient carefully and frequently for signs of bleeding; take vital signs (avoiding automatic BP cuffs); observe any invaded sites at least every 15 minutes (e.g., sheaths, IV sites, cutdowns, punctures, Foleys, NGs); watch for hematuria, hematemesis, hemoptysis, bloody stool, petechiae, hematoma, flank pain, muscle weakness; and do neuro checks every hour. Continue until clotting functions move toward normal. ■ Use care in handling patient; avoid arterial puncture, venipuncture, and IM injection. Use extreme precautionary methods and only compressible sites if these procedures are absolutely necessary. Apply pressure for 30 minutes to any invaded site and then apply pressure dressings. Saline or heparin locks are suggested to facilitate blood draws. ■ Minimize use of urinary catheters, nasotracheal intubation, nasogastric tubes, and automatic blood pressure cuffs. Discontinue heparin after PCI and remove sheath no sooner than 2 hours and no later than 6 hours after heparin is discontinued (aPTT must be at or less than 50 seconds or ACT at or less than 175 seconds). After removal, apply pressure to the femoral artery for at least 30 minutes. When hemostasis is confirmed, apply a pressure dressing. Maintain strict bed rest for at least 6 to 8 hours after sheath removal and/or abciximab is discontinued or 4 hours after heparin is discontinued, whichever is later. ■ Throughout process medicate as needed for back or groin pain and nausea or vomiting. ■ Remove pressure dressing before ambulation. ■ In the event of serious, uncontrolled bleeding or the need for emergency surgery, discontinue abciximab. Platelet function may be partly restored with platelet transfusions. ■ See Precautions, Drug/Lab Interactions, and Antidote.
Patient education:
Compliance with all measures to minimize bleeding (e.g., strict bed rest, positioning) is imperative. ■ Avoid use of razors, toothbrushes, and other sharp items. ■ Use caution while moving to avoid excessive bumping. ■ Report all episodes of bleeding and apply local pressure if indicated. ■ Expect oozing from IV sites.
Maternal/child:
Category C: use only if clearly needed and with extreme caution. ■ Safety for use during breast-feeding not established. Not known if it is secreted in breast milk; use extreme caution; probably best to postpone breast-feeding until bleeding time approaches normal. ■ Safety and effectiveness for use in pediatric patients not established.
Elderly:
No overall difference in safety or efficacy observed in patients between 65 and 75 years of age as compared with younger patients. Insufficient data to determine whether patients age 75 or older respond differently. ■ Increased risk of major bleeding complications if weight less than 75 kg; see Precautions. ■ Consider age-related organ impairment, concomitant disease, or drug therapy; may also increase risk of bleeding.
Drug/lab interactions
Formal drug interaction studies have not been conducted. ■ Use with extreme caution with other drugs that affect hemostasis (e.g., thrombolytics [e.g., alteplase (tPA), streptokinase], anticoagulants [e.g., heparin, warfarin (Coumadin)], NSAIDs [e.g., ibuprofen (Advil, Motrin), naproxen (Aleve, Naprosyn)], platelet aggregation inhibitors [e.g., clopidogrel (Plavix), dipyridamole (Persantine), ticlopidine (Ticlid)] and other glycoprotein GPIIb/IIIa receptor antagonists [e.g., eptifibatide (Integrilin), tirofiban (Aggrastat)], and selected antibiotics [e.g., cefotetan]). ■ Dextran solutions increased the risk of major bleeding events when used concurrently with abciximab; see Contraindications. ■ HACA titer may precipitate an acute hypersensitivity reaction with other diagnostic or therapeutic monoclonal antibodies (e.g., muromonab-CD3). ■ Has been administered to patients with ischemic heart disease treated concomitantly with heparin, warfarin, beta-adrenergic receptor blockers (e.g., metoprolol [Lopressor]), calcium channel antagonists (e.g., diltiazem [Cardizem]), angiotensin-converting enzyme inhibitors (e.g., enalapril [Vasotec]), nitrates, ticlopidine (Ticlid), and aspirin.
Side effects
May cause major bleeding incidents (e.g., femoral artery or other access site, intracranial hemorrhage, spontaneous gross hematuria and other GU bleeds, spontaneous hematemesis and other GI bleeds, pulmonary alveolar hemorrhage, retroperitoneal bleeding). Decreases in hemoglobin greater than 5 Gm/dL or intracranial hemorrhage were defined as major during trials. Thrombocytopenia is common and may require platelet transfusion. Abdominal pain, back pain, bradycardia, chest pain, headache, hypotension, nausea, peripheral edema, positive HACA response, hypersensitivity reactions (including anaphylaxis), puncture site pain, and vomiting may occur. Other side effects that may occur are anemia, arrhythmias (e.g., atrial fibrillation/flutter, bradycardia, complete AV block, supraventricular tachycardia, ventricular PVCs, tachycardia, or fibrillation), confusion, hyperesthesia, intermittent claudication, leukocytosis, limb embolism, pericardial effusion, pleural effusion or pleurisy, pneumonia, pulmonary edema, pulmonary embolism, and visual disturbances.
Antidote
Stop the infusions of abciximab and heparin if any serious bleeding not controllable with pressure occurs. Stop infusion in patients with failed PCI. Stop infusion if a hypersensitivity reaction occurs. Treat hypersensitivity reactions as indicated; may require epinephrine, airway management, oxygen, IV fluids, antihistamines (e.g., diphenhydramine [Benadryl]), corticosteroids (e.g., hydrocortisone sodium succinate [Solu-Cortef]), and pressor amines (e.g., dopamine). Keep physician informed. If an acute platelet decrease occurs (less than 100,000/mm3 or a decrease of at least 25% from pretreatment value), obtain additional platelet counts in separate tubes containing ethylenediaminetetraacetic acid (EDTA), citrate, and heparin. This is to exclude pseudothrombocytopenia due to anticoagulant interaction. If true thrombocytopenia is verified, discontinue abciximab immediately. Platelet transfusions may be required. Heparin and aspirin should also be avoided if the platelet count drops below 60,000/mm3.
Acetaminophen 
(ah-SEAT-ah-MIN-oh-fen)
Ofirmev
Antipyretic
Analgesic
pH 5.5
Usual dose
May be given as a single or repeated dose. Minimum dosing interval is 4 hours. No dose adjustment is necessary when converting from oral to IV dosing.
Care must be taken to avoid dosing errors, which could result in accidental overdose and death. In particular, be careful to ensure that:
• Dose in milligrams and milliliters is not confused
• Dosing is based on weight for patients under 50 kg
• Infusion pump is programmed properly
• Maximum single dose and the maximum total daily dose of acetaminophen from all sources (i.e., IV, oral, and rectal) and all products containing acetaminophen do not exceed maximum limits.
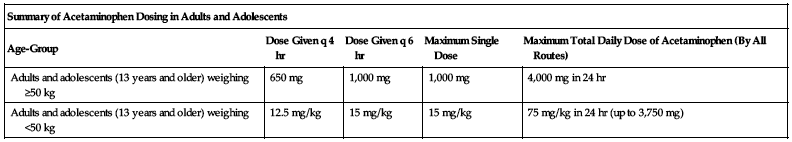
| Summary of Acetaminophen Dosing in Adults and Adolescents | ||||
| Age-Group | Dose Given q 4 hr | Dose Given q 6 hr | Maximum Single Dose | Maximum Total Daily Dose of Acetaminophen (By All Routes) |
| Adults and adolescents (13 years and older) weighing ≥50 kg | 650 mg | 1,000 mg | 1,000 mg | 4,000 mg in 24 hr |
| Adults and adolescents (13 years and older) weighing <50 kg | 12.5 mg/kg | 15 mg/kg | 15 mg/kg | 75 mg/kg in 24 hr (up to 3,750 mg) |
Pediatric dose
Pediatric patients 2 to 12 years of age:
15 mg/kg every 6 hours or 12.5 mg/kg every 4 hours. Do not exceed a maximum single dose of 15 mg/kg or a maximum daily dose of 75 mg/kg/day. See comments under Usual Dose.
Dose adjustments
A reduced total daily dose of acetaminophen may be appropriate in patients with hepatic impairment or active liver disease. ■ A reduced total daily dose and longer dosing intervals may be appropriate in patients with a CrCl less than or equal to 30 mL/min.
Dilution
Available in a single-use vial containing 1,000 mg/100 mL (10 mg/mL) of acetaminophen. For adults and adolescent patients weighing 50 kg or more requiring a 1,000-mg dose, administer the dose by inserting a vented intravenous set through the septum of the 100-mL vial. Doses less than 1,000 mg should be withdrawn from the vial and placed into a separate empty container before administration to avoid inadvertent administration of an overdose. Withdraw appropriate dose (650 mg or weight-based) from 100-mL vial and place in an empty container (e.g., syringe, glass bottle, plastic intravenous container) for intravenous infusion.
Filter:
Information not available.
Storage:
Store unopened vial at CRT. Do not refrigerate or freeze. Discard 6 hours after entry into vial or transfer into an empty container. Single-use vial. Discard any unused solution.
Compatibility
Manufacturer states, “Do not add other medications to solution. Incompatible with diazepam and chlorpromazine. Do not administer simultaneously.”
One source suggests the following compatibilities.
Solutions:
D5W, NS.
Y-site:
Buprenorphine (Buprenex), butorphanol (Stadol), cefoxitin (Mefoxin), ceftriaxone (Rocephin), clindamycin (Cleocin), D5LR, D5NS, D10W, dexamethasone (Decadron), diphenhydramine (Benadryl), dolasetron (Anzemet), droperidol (Inapsine), fentanyl, granisetron (Kytril), heparin, hydrocortisone sodium succinate (Solu-Cortef), hydromorphone (Dilaudid), ketorolac (Toradol), lidocaine, lorazepam (Ativan), LR, mannitol (Osmitrol), meperidine (Demerol), methylprednisolone sodium succinate (Solu-Medrol), metoclopramide (Reglan), midazolam (Versed), morphine, nalbuphine, ondansetron (Zofran), piperacillin/tazobactam (Zosyn), potassium chloride, prochlorperazine (Compazine), ranitidine (Zantac), sufentanil (Sufenta), vancomycin.
Rate of administration
Administer as an infusion, equally distributed over 15 minutes. Pediatric doses up to 600 mg may be drawn up into a syringe and delivered via a syringe pump.
Actions
A nonsalicylate antipyretic and a nonopioid analgesic agent. Exact mechanism of action is unknown but is thought to act through central actions. Widely distributed into most tissues except fat. Low protein binding (10% to 25%). Half-life is approximately 2 to 3 hours. Metabolized in the liver via three different pathways. Metabolites excreted in the urine.
Indications and uses
Management of mild to moderate pain. ■ Management of moderate to severe pain with adjunctive opioid analgesics. ■ Reduction of fever.
Contraindications
Known hypersensitivity to acetaminophen or to any components of the IV formulation. ■ Patients with severe hepatic impairment or severe active liver disease.
Precautions
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most cases of liver injury are associated with the use of acetaminophen at doses that exceed the maximum daily limits and often involve more than one acetaminophen-containing product. Do not exceed the maximum recommended daily dose. ■ Use with caution in patients with hepatic impairment or active hepatic disease, alcoholism, chronic malnutrition, severe hypovolemia (e.g., due to dehydration or blood loss), or severe renal impairment (CrCl less than or equal to 30 mL/min). ■ Serious skin reactions such as acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported rarely. ■ Hypersensitivity and anaphylactic reactions have been reported. ■ Care must be taken when prescribing, preparing, or administering acetaminophen to avoid dosing errors, which could result in accidental overdose and death; see Usual Dose. ■ Antipyretic effects may mask fever in patients treated for postsurgical pain.
Monitor:
Monitor for S/S of hypersensitivity reaction (e.g., respiratory distress; pruritus; rash; swelling of the face, mouth, and throat; urticaria). ■ Monitor for S/S of serious skin reactions. ■ Baseline SCr and liver function tests may be indicated.
Maternal/child:
Category C: epidemiologic data on oral acetaminophen use in pregnant women show no increased risk of major congenital malformations. Safety of IV formulation for use in pregnancy not established. Use only if clearly needed. ■ Assess benefit versus risk before use during labor and delivery. ■ Safety for use in breast-feeding not established. Acetaminophen is secreted in human milk in small quantities after oral administration. Use caution. ■ Safety and effectiveness for treatment of acute pain or fever has not been studied in pediatric patients less than 2 years of age.
Elderly:
No overall differences in safety and efficacy were observed between older and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Drug/lab interactions
Substances that induce or regulate hepatic cytochrome enzyme CYP2E1 (e.g., ethanol, isoniazid) may alter the metabolism of acetaminophen and increase its hepatotoxic potential. Effects have not been studied. ■ Ethanol may induce hepatic cytochromes and may act as a competitive inhibitor of the metabolism of acetaminophen. ■ Chronic acetaminophen doses of 4,000 mg/day may cause an increase in INR in patients stabilized on warfarin. Effect of short-term use on INR has not been studied. Monitoring of INR recommended. ■ Many available analgesics contain acetaminophen in combination with another analgesic (e.g., hydrocodone/acetaminophen [Vicodin, Norco], oxycodone/acetaminophen [Percocet]). Over-the-counter cold and allergy preparations may also contain acetaminophen in combination with other active ingredients. Monitor total daily dose of acetaminophen coming from all possible sources.
Side effects
Adult patients:
The most common adverse reactions were headache, insomnia, nausea, and vomiting. Less frequently reported side effects included anxiety, dyspnea, fatigue, hypersensitivity reaction, hypertension, hypokalemia, hypotension, increased aspartate aminotransferase, infusion site pain, muscle spasms, peripheral edema, and trismus.
Pediatric patients:
The most common adverse reactions were agitation, atelectasis, constipation, nausea, pruritus, and vomiting. Less commonly reported side effects included abdominal pain, anemia, diarrhea, fever, headache, hypersensitivity reaction, hypertension, hypervolemia, hypoalbuminemia, hypokalemia, hypomagnesemia, hypophosphatemia, hypotension, hypoxia, increased hepatic enzymes, injection site pain, insomnia, muscle spasm, oliguria, pain in extremities, periorbital edema, peripheral edema, pleural effusion, pulmonary edema, rash, stridor, tachycardia, and wheezing.
Overdose:
Hepatic necrosis, renal tubular necrosis, hypoglycemic coma, and thrombocytopenia.
Post-marketing:
Hypersensitivity and anaphylaxis (e.g., respiratory distress; pruritus; rash; swelling of the face, mouth, and throat; urticaria).
Antidote
Notify the physician of significant side effects. Discontinue immediately at the first appearance of skin rash or any other sign of hypersensitivity. Treat as indicated (e.g., diphenhydramine, epinephrine, albuterol). Resuscitate as necessary. If an acetaminophen overdose is suspected, obtain a serum acetaminophen level and baseline liver function studies. N-acetylcysteine antidote may be indicated. See acetylcysteine monograph. Contact a regional poison control center for additional information.
Acetazolamide sodium
(ah-set-ah-ZOE-la-myd SO-dee-um)
Diamox
Antiglaucoma
Anticonvulsant
Diuretic
Urinary alkalinizer
pH 9.2
Usual dose
Antiglaucoma agent:
250 mg to 1 Gm/24 hr. May be given as 250-mg doses at 4- to 6-hour intervals. In the treatment of secondary glaucoma and in the preoperative treatment of some cases of acute congestive (closed-angle) glaucoma, the preferred dose is 250 mg every 4 hours. In acute cases, to rapidly lower intraocular pressure, an initial single dose of 500 mg followed by 125 to 250 mg at 4-hour intervals may be given.
Edema of congestive heart failure or drug therapy:
250 to 375 mg or 5 mg/kg of body weight as a single dose daily; when loss of edematous fluid stops, reduce to every other day or give for 2 days followed by a day of rest.
Anticonvulsant:
Adults and pediatric patients:
Dose in epilepsy may range from 8 to 30 mg/kg/24 hr in divided doses every 6 to 12 hours (2 to 7.5 mg/kg every 6 hours or 4 to 15 mg/kg every 12 hours). Reduce initial daily dose when given with other anticonvulsants.
Urinary alkalinization:
Adults and pediatric patients:
5 mg/kg/dose every 8 to 12 hours.
Pediatric dose
See Maternal/Child.
Acute antiglaucoma agent:
5 to 10 mg/kg every 6 hours. Do not exceed 1,000 mg/24 hr.
Edema of congestive heart failure or drug therapy:
5 mg/kg as a single dose daily or every other day; see comment under Usual Dose. Do not exceed 1,000 mg/24 hr.
Slowly progressive hydrocephalus in infants 2 weeks to 10 months (unlabeled):
20 mg/kg/24 hr in equally divided doses every 8 hours (8.3 mg/kg every 8 hours). Up to 100 mg/kg/24 hr or a maximum dose of 2 Gm/24 hr has been used.
Dose adjustments
Reduced dose required when introducing acetazolamide into a treatment regimen with other anticonvulsants. ■ Administer every 12 hours in patients with a CrCl from 10 to 50 mL/min. Avoid use in patients with a CrCl less than 10 mL/min (ineffective).
Dilution
Each 500 mg should be diluted in 5 mL SWFI. May then be given by IV injection or added to standard IV fluids. IM administration not recommended.
Storage:
Reconstituted solution stable for 12 hours at RT or 3 days refrigerated.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Additive:
Ranitidine (Zantac).
Y-site:
Diltiazem (Cardizem).
Rate of administration
500 mg or fraction thereof over at least 1 minute or added to IV fluids to be given over 4 to 8 hours.
Actions
A potent carbonic anhydrase inhibitor and nonbacteriostatic sulfonamide, acetazolamide depresses the tubular reabsorption of sodium, potassium, and bicarbonate. Excreted unchanged in the urine, producing diuresis, alkalinization of the urine, and a mild degree of metabolic acidosis.
Indications and uses
Adjunctive treatment of edema due to congestive heart failure, drug-induced edema, centrencephalic epilepsies (petit mal, unlocalized seizures), chronic simple (open-angle) glaucoma, and secondary glaucoma, and preoperatively in acute angle-closure glaucoma when delay of surgery is desired to lower intraocular pressure. ■ Used orally for acute mountain sickness.
Unlabeled uses:
Metabolic alkalosis, urine alkalinization, respiratory stimulant in COPD.
Contraindications
Depressed sodium and potassium levels, hyperchloremic acidosis, marked kidney or liver disease, adrenocortical insufficiency, hypersensitivity to acetazolamide or any of its components. Long-term use contraindicated in some glaucomas.
Precautions
Chemically related to sulfonamides; may cause serious reactions in sensitive patients. ■ May be alternated with other diuretics to achieve maximum effect. ■ Greater diuretic action is achieved by skipping a day of treatment rather than increasing dose; failure in therapy may be due to overdose or too-frequent dosage. ■ IM administration not recommended. Administration by IV injection is preferred. ■ Use with caution in impaired respiratory function (e.g., pulmonary disease, edema, infection, obstruction); may cause severe respiratory acidosis. ■ Potassium excretion is proportional to diuresis. Hypokalemia may result from diuresis or with severe cirrhosis. ■ Introduce or withdraw gradually when used as an anticonvulsant.
Monitor:
Obtain baseline CBC and platelet count before use and monitor during therapy. ■ Periodic monitoring of electrolytes is recommended.
Patient education:
Consider birth control options.
Maternal/child:
Category C: has been shown to be teratogenic in animal studies. Use during pregnancy only if potential benefit justifies potential risks to the fetus. ■ Discontinue breast-feeding or discontinue acetazolamide. ■ Safety for use in pediatric patients not established, but no problems are documented.
Elderly:
Use caution; no documented problems, but age-related renal impairment may be a factor.
Drug/lab interactions
May cause hypokalemia with concurrent use of steroids. ■ Hypokalemia may cause toxicity and fatal cardiac arrhythmias with digoxin or interfere with insulin or oral antidiabetic agent response, thus causing hyperglycemia. ■ Alkalinization of urine potentiates amphetamines, ephedrine, flecainide (Tambocor), methenamine, procainamide, pseudoephedrine (Sudafed), quinidine, and tricyclic antidepressants (e.g., amitriptyline [Elavil]) by decreasing rate of excretion. ■ May decrease response to lithium, methotrexate, some antidepressants, phenobarbital, salicylates, and urinary anti-infectives by increasing rate of excretion. ■ Metabolic acidosis induced by acetazolamide may potentiate salicylate toxicity (anorexia, tachypnea, lethargy, coma, and death can occur with high-dose aspirin). ■ Alkalinity may cause false-positive urinary protein and possibly urinary steroid tests. ■ May depress iodine uptake by the thyroid.
Side effects
Minimal with short-term therapy. Respond to symptomatic treatment or withdrawal of drug: acidosis, anorexia, bone marrow suppression, confusion, crystalluria, drowsiness, fever, hemolytic anemia, hypokalemia (ECG changes, fatigue, muscle weakness, vomiting), paresthesias, photosensitivity, polyuria, rash, renal calculus, thrombocytopenic purpura.
Antidote
Notify physician of any adverse effects and discontinue drug if necessary. Treat hypersensitivity reactions as indicated; may require epinephrine, airway management, oxygen, IV fluids, antihistamines (e.g., diphenhydramine [Benadryl]), corticosteroids (e.g., hydrocortisone sodium succinate [Solu-Cortef]), and pressor amines (e.g., dopamine). Moderately dialyzable (20% to 40%).
Acetylcysteine injection  *
*
(ah-see-till-SIS-tay-een in-JEK-shun)
Acetadote
Antidote
pH 6 to 7.5
Usual dose (adult and pediatric)
Assess the potential risk of hepatotoxicity by determining plasma or serum acetaminophen concentrations as early as possible but no sooner than 4 hours following an acute overdose. Acetylcysteine may be withheld until acetaminophen assay results are available as long as initiation of treatment is not delayed beyond 8 hours postingestion; see Precautions and Monitor. Total dose equals 300 mg/kg given as three separate doses and administered over 21 hours. Total volume administered for patients less than 40 kg and for those requiring fluid restriction can be adjusted as clinically needed; see Dosing chart for patients who weigh from 5 to 20 kg and from 21 to 40 kg. Consider osmolarity; see Dilution and Precautions.
Distribute doses as indicated in the following guidelines:
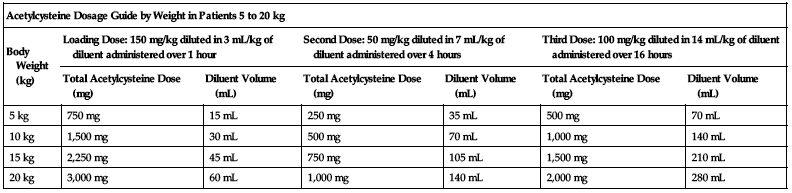
Dosing for patients who weigh 5 to 20 kg:
Loading dose:
150 mg/kg diluted in 3 mL/kg of diluent administered over 1 hour.
Second dose:
50 mg/kg diluted in 7 mL/kg of diluent administered over 4 hours.
Third dose:
100 mg/kg diluted in 14 mL/kg of diluent administered over 16 hours.
| Acetylcysteine Dosage Guide by Weight in Patients 5 to 20 kg | ||||||
| Body Weight (kg) | Loading Dose: 150 mg/kg diluted in 3 mL/kg of diluent administered over 1 hour | Second Dose: 50 mg/kg diluted in 7 mL/kg of diluent administered over 4 hours | Third Dose: 100 mg/kg diluted in 14 mL/kg of diluent administered over 16 hours | |||
| Total Acetylcysteine Dose (mg) | Diluent Volume (mL) | Total Acetylcysteine Dose (mg) | Diluent Volume (mL) | Total Acetylcysteine Dose (mg) | Diluent Volume (mL) | |
| 5 kg | 750 mg | 15 mL | 250 mg | 35 mL | 500 mg | 70 mL |
| 10 kg | 1,500 mg | 30 mL | 500 mg | 70 mL | 1,000 mg | 140 mL |
| 15 kg | 2,250 mg | 45 mL | 750 mg | 105 mL | 1,500 mg | 210 mL |
| 20 kg | 3,000 mg | 60 mL | 1,000 mg | 140 mL | 2,000 mg | 280 mL |
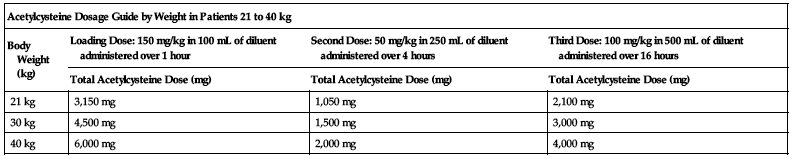
Dosing for patients who weigh 21 to 40 kg:
Loading dose:
150 mg/kg diluted in 100 mL of diluent administered over 1 hour.
Second dose:
50 mg/kg diluted in 250 mL of diluent administered over 4 hours.
Third dose:
100 mg/kg diluted in 500 mL of diluent administered over 16 hours.
| Acetylcysteine Dosage Guide by Weight in Patients 21 to 40 kg | |||
| Body Weight (kg) | Loading Dose: 150 mg/kg in 100 mL of diluent administered over 1 hour | Second Dose: 50 mg/kg in 250 mL of diluent administered over 4 hours | Third Dose: 100 mg/kg in 500 mL of diluent administered over 16 hours |
| Total Acetylcysteine Dose (mg) | Total Acetylcysteine Dose (mg) | Total Acetylcysteine Dose (mg) | |
| 21 kg | 3,150 mg | 1,050 mg | 2,100 mg |
| 30 kg | 4,500 mg | 1,500 mg | 3,000 mg |
| 40 kg | 6,000 mg | 2,000 mg | 4,000 mg |
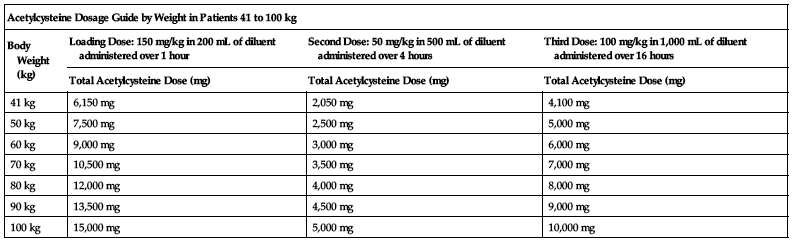
Dosing for patients who weigh 41 to 100 kg:
Loading dose:
150 mg/kg diluted in 200 mL of diluent administered over 1 hour.
Second dose:
50 mg/kg diluted in 500 mL of diluent administered over 4 hours.
Third dose:
100 mg/kg diluted in 1,000 mL of diluent administered over 16 hours.
| Acetylcysteine Dosage Guide by Weight in Patients 41 to 100 kg | |||
| Body Weight (kg) | Loading Dose: 150 mg/kg in 200 mL of diluent administered over 1 hour | Second Dose: 50 mg/kg in 500 mL of diluent administered over 4 hours | Third Dose: 100 mg/kg in 1,000 mL of diluent administered over 16 hours |
| Total Acetylcysteine Dose (mg) | Total Acetylcysteine Dose (mg) | Total Acetylcysteine Dose (mg) | |
| 41 kg | 6,150 mg | 2,050 mg | 4,100 mg |
| 50 kg | 7,500 mg | 2,500 mg | 5,000 mg |
| 60 kg | 9,000 mg | 3,000 mg | 6,000 mg |
| 70 kg | 10,500 mg | 3,500 mg | 7,000 mg |
| 80 kg | 12,000 mg | 4,000 mg | 8,000 mg |
| 90 kg | 13,500 mg | 4,500 mg | 9,000 mg |
| 100 kg | 15,000 mg | 5,000 mg | 10,000 mg |
Dosing for patients who weigh more than 100 kg:
Limited data available. Manufacturer recommends:
Loading dose:
15,000 mg diluted in 200 mL administered over 1 hour.
Second dose:
5,000 mg diluted in 500 mL of diluent administered over 4 hours.
Third dose:
10,000 mg diluted in 1,000 mL of diluent administered over 16 hours.
Dose adjustments
Therapy extending beyond 21 hours may be considered in rare cases such as suspected massive overdose, concomitant ingestion of other substances, or in patients with pre-existing liver disease. In these cases, absorption and/or half-life of acetaminophen may be prolonged. Obtain acetaminophen levels and ALT/AST and INR before the end of the 21-hour infusion. If acetaminophen levels are still detectable, or in cases in which ALT/AST is still increasing or INR remains elevated, continue the infusion and contact a regional poison control center. ■ Specific information and/or recommendations are not available for patients with impaired hepatic or renal function.
Dilution
May be diluted in D5W, 1/2NS, or SWFI. See Usual Dose for dilution guidelines based on weight. Total volume administered should be adjusted for patients less than 40 kg or for those requiring fluid restriction. Hyponatremia and seizures may result from large volumes in small children. ■ A hyperosmolar solution. Caution is advised when the diluent volume is decreased; hyperosmolarity of the solution is increased as shown in the following chart.
| Acetylcysteine Concentration and Osmolarity | |||
| Acetylcysteine Concentration (mg/mL) | Osmolarity in ½NS (mOsmol/L) | Osmolarity in D5W (mOsmol/L) | Osmolarity in SWFI (mOsmol/L) |
| 7 mg/mL | 245 mOsmol/L | 343 mOsmol/L | 91 mOsmol/L* |
| 24 mg/mL | 466 mOsmol/L | 564 mOsmol/L | 312 mOsmol/L |

*Osmolarity should be adjusted to a physiologically safe level (generally not less than 150 mOsmol/L in children).
Color of acetylcysteine may change from colorless to a slight pink or purple once the stopper is punctured; quality is not affected.
Filters:
Data not available and use is not required by manufacturer. Studies on the use of filters are planned.
Storage:
Store unopened vials at CRT. Diluted solution is stable for 24 hours at CRT. Do not use previously opened vials for IV administration. Discard unused portions.
Compatibility
Manufacturer states, “Compatible with D5W, 1/2NS, and SWFI.”
Rate of administration
Usual total infusion time of all 3 doses is 21 hours. Rate reduction may be required to manage S/S of infusion reactions; see Monitor and Antidote.
Loading dose:
An infusion evenly distributed over 60 minutes.
Second dose:
An infusion evenly distributed over 4 hours.
Third dose:
An infusion evenly distributed over 16 hours.
Actions
Protects the liver by maintaining or restoring the glutathione levels (metabolites formed after an overdose of acetaminophen may deplete the hepatic stores of glutathione and cause binding of the metabolite to protein molecules within the hepatocyte, resulting in cellular necrosis). It may also act by forming an alternate compound and detoxifying the reactive metabolite. Half-life is approximately 5.6 hours. Metabolizes to various compounds. Crosses the placental barrier. Some excretion in urine.
Indications and uses
To prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen. Overdose incidences are divided into two types: (1) acute ingestion, or (2) repeated supratherapeutic ingestion (RSI).
Contraindications
Known hypersensitivity to acetylcysteine or any of its components.
Precautions
Should be administered in a facility equipped to monitor the patient and respond to any medical emergency. ■ Most effective against severe hepatic injury when administered within 8 hours of ingestion. Administration before 4 hours does not allow enough time to determine an actual need for treatment with acetylcysteine; serum levels drawn before 4 hours have passed may be misleading. Effectiveness diminishes gradually after 8 hours. Should be administered if 24 hours or less has passed since ingestion, because the reported time of ingestion may not be correct and it does not appear to worsen the patient’s condition. ■ Total volume administered should be adjusted for patients less than 40 kg and for patients requiring fluid restriction. If volume is not adjusted, fluid overload can occur, potentially resulting in hyponatremia, seizure, and death; see Usual Dose. ■ Anaphylactoid reactions have been reported and usually occur soon after initiation of the infusion. Use caution in patients with asthma or a history of bronchospasm. Death occurred in a patient with asthma. ■ Acute flushing and erythema of the skin may occur. These reactions usually occur within 30 to 60 minutes of beginning the infusion and often resolve spontaneously despite continued infusion. ■ Clearance decreased and half-life prolonged in patients with various stages of liver damage (Child-Pugh scores of 5 to 13). ■ The Rumack-Matthew nomogram does not apply to patients with repeated supratherapeutic ingestion (RSI), which is defined as ingestion of acetaminophen at doses higher than those recommended for extended periods of time. For treatment information, see prescribing information for a professional assistance line for acetaminophen overdose, or contact a regional poison control center. ■ The Rumack-Matthew nomogram may underestimate the risk for hepatotoxicity in some patients with risk factors such as chronic alcoholism, malnutrition, or CYP2E1 enzyme-inducing drugs (e.g., isoniazid [INH]). ■ Vial stopper does not contain natural rubber latex. ■ See Monitor and Antidote.
Monitor:
Acute ingestion of 150 mg/kg or more of acetaminophen may result in hepatic toxicity. Obtain baseline hepatic function studies and monitor throughout detoxification process.
Preferred method of treatment:
Estimate time of acetaminophen ingestion. If less than 24 hours since overdose, draw serum for an acetaminophen level at 4 hours postingestion or as soon as possible thereafter to clarify the need for intervention with acetylcysteine. The serum acetaminophen level should be evaluated on the Rumack-Matthew nomogram to determine the probability of toxicity (see package insert for copy of nomogram). ■ If serum acetaminophen level is below the treatment line on the nomogram, discontinue the acetylcysteine if initiated as a precaution. If the plasma level is above the treatment line on the nomogram, initiate or continue treatment.
Secondary options for treatment:
If serum acetaminophen levels are not available within 8 hours, initiate treatment. Do not delay treatment more than 8 hours postingestion. ■ If time of ingestion is unknown or if the patient is unreliable, consider empiric initiation of acetylcysteine treatment. ■ If a serum acetaminophen level is not available or cannot be interpreted and less than 24 hours has elapsed since ingestion, administer acetylcysteine regardless of the quantity reported to have been ingested.
All treatment options:
Obtain serum acetaminophen level and baseline ALT, AST, bilirubin, blood glucose, BUN, electrolytes, PT, and SCr. Monitor as indicated by results. ■ Monitor BP and HR before, during, and after the infusion. ■ Evaluate serum acetaminophen level on the Rumack-Matthew nomogram. ■ Infusion reactions may begin with acute flushing and erythema of the skin. May resolve spontaneously despite continued infusion of acetylcysteine or may progress to an acute hypersensitivity reaction and/or anaphylaxis. Observe continuously for initial S/S of a hypersensitivity reaction (e.g., hypotension, rash, shortness of breath, wheezing). ■ In suspected toxicity resulting from the extended-release acetaminophen preparation, an acetaminophen level drawn fewer than 8 hours postingestion may be misleading. Draw a second level at 4 to 6 hours after the initial level. If either acetaminophen level falls above the toxicity line, acetylcysteine treatment should be initiated. ■ See Precautions and Antidote.
Patient education:
Report S/S of hypersensitivity promptly (e.g., flushing, itching, shortness of breath, feeling of faintness).
Maternal/child:
Category B: use during pregnancy only if clearly needed. ■ Use caution; safety for use during breast-feeding not established; effects unknown. After 30 hours, acetylcysteine should be cleared from maternal blood, and breast-feeding can be resumed. ■ No adequate or well-controlled studies in pediatric patients, but it has been used. Efficacy appears to be similar to that seen in adults; see Side Effects. ■ Administered to a small number of preterm infants during clinical studies (a mean rate of 4.2 mg/kg/hr for 24 hours); half-life prolonged to approximately 11 hours in these newborns. ■ Acetylcysteine was measurable in the circulation and cord blood of three newborns whose mothers were treated for acetaminophen overdose. No adverse side effects were noted, and none of the infants had evidence of acetaminophen poisoning.
Elderly:
Differences in response compared with younger adults not known.
Drug/lab interactions
Drug-drug interaction studies have not been done.
Side effects
Adult and pediatric patients:
Pruritus, rash, and urticaria have been reported most frequently and most commonly occur during the initial loading dose. Other reported side effects include anaphylactoid reactions, angioedema, dyspepsia, dysphoria (abnormal thinking or confusion), dyspnea, edema, erythema of the skin, eye pain, facial flushing, gait disturbances, hypotension, nausea and vomiting, palmar erythema, respiratory symptoms (e.g., bronchospasm, chest tightness, cough, respiratory distress, shortness of breath, stridor, wheezing), sweating, syncope, tachycardia, vasodilation.
Overdose:
S/S of acute toxicity in animals included ataxia, convulsions, cyanosis, hypoactivity, labored respiration, and loss of righting reflex.
Antidote
Keep physician informed of all side effects. Flushing and erythema of the skin are expected. If other symptoms of a hypersensitivity reaction occur (e.g., bronchospasm, dyspnea, hypotension, wheezing), discontinue acetylcysteine and treat with diphenhydramine (Benadryl) or epinephrine as indicated. After symptoms subside, the infusion may be carefully resumed. If S/S of hypersensitivity recur, discontinue infusion permanently and consider alternate treatments. Contact a regional poison control center for possible treatment alternatives.
Acyclovir
(ay-SYE-kloh-veer)
Zovirax
Antiviral
pH 10.5 to 11.6
Usual dose
In all situations for adults, adolescents, children, and neonates, do not exceed a maximum dose of 20 mg/kg every 8 hours.
Adults and adolescents (12 years of age and older): Herpes simplex infections; mucosal and cutaneous HSV infections in immunocompromised patients:
5 mg/kg of body weight every 8 hours for 7 days.
Severe initial clinical episodes of herpes genitalis:
5 mg/kg every 8 hours for 5 days.
Herpes simplex encephalitis:
10 mg/kg every 8 hours for 10 days.
Herpes zoster infections (shingles) in immunocompromised patients:
10 mg/kg every 8 hours for 7 days.
Pediatric dose
Pediatric patients under 12 years of age: Herpes simplex infections; mucosal and cutaneous HSV infections in immunocompromised patients:
10 mg/kg every 8 hours for 7 days.
Herpes simplex encephalitis:
Pediatric patients over 3 months of age:
20 mg/kg every 8 hours for 10 days.
Herpes zoster infections (shingles) in immunocompromised patients:
20 mg/kg every 8 hours for 7 days.
Neonatal dose
Neonatal herpes simplex virus infections:
Birth to 3 months:
10 mg/kg every 8 hours for 10 days. Doses of 15 mg/kg to 20 mg/kg every 8 hours have been used for up to 14 to 21 days; safety and efficacy have not been established.
Dose adjustments
Calculate dose by ideal body weight in obese individuals. ■ Reduced dose may be indicated in the elderly based on the potential for decreased renal function and concomitant disease or drug therapy. ■ In adults and pediatric patients with impaired renal function, reduce dose and/or adjust dosing interval based on CrCl as indicated in the following chart.
| Acyclovir Dosage Adjustments for Adults and Pediatric Patients with Renal Impairment | ||
| Creatinine Clearance (mL/min per 1.73 M2) | Percent of Recommended Dose | Dosing Interval (hours) |
| >50 mL/min | 100% | Every 8 hr |
| 25-50 mL/min | 100% | Every 12 hr |
| 10-25 mL/min | 100% | Every 24 hr |
| 0-10 mL/min | 50% | Every 24 hr |
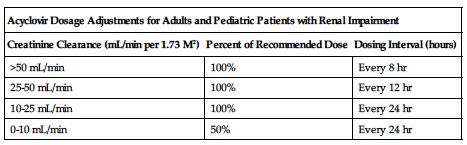
Plasma concentrations decrease with hemodialysis; adjustment of the dosing schedule is recommended so that an additional dose is administered after each dialysis. No supplemental dose is indicated in peritoneal dialysis after adjustment of the dosing interval.
Dilution
Initially dissolve each 500 mg with 10 mL SWFI (1,000 mg with 20 mL). Concentration equals 50 mg/mL. Do not use bacteriostatic water for injection (BWFI); will cause precipitation. Shake well to dissolve completely. Also available in liquid vials. Withdraw the desired dose and further dilute in an amount of solution to provide a concentration less than 7 mg/mL (70-kg adult at 5 mg/kg equals 350 mg dissolved in a total of 100 mL of solution equals 3.5 mg/mL). Compatible with most standard electrolyte and glucose infusion solutions.
Filters:
No data available from manufacturer.
Storage:
Store unopened vials at CRT. Use reconstituted solution within 12 hours; high pH may result in etching of glass vial surface after 12 hours. Use solution fully diluted for administration within 24 hours. Manufacturer will supply data showing stability for longer periods under specific conditions.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer lists bacteriostatic water for injection (BWFI) as incompatible. Dilution in biologic or colloidal fluids (e.g., blood products, protein solutions) is not recommended.
One source suggests the following compatibilities:
Additive:
Fluconazole (Diflucan), meropenem (Merrem IV).
Y-site:
Allopurinol (Aloprim), amikacin, amphotericin B cholesteryl (Amphotec), ampicillin, anidulafungin (Eraxis), caspofungin (Cancidas), cefazolin (Ancef), cefotaxime (Claforan), cefoxitin (Mefoxin), ceftaroline (Teflaro), ceftazidime (Fortaz), ceftriaxone (Rocephin), cefuroxime (Zinacef), chloramphenicol (Chloromycetin), cisatracurium (Nimbex), clindamycin (Cleocin), dexamethasone (Decadron), diltiazem (Cardizem), dimenhydrinate, diphenhydramine (Benadryl), docetaxel (Taxotere), doripenem (Doribax), doxorubicin liposomal (Doxil), doxycycline, droperidol (Inapsine), erythromycin (Erythrocin), etoposide phosphate (Etopophos), famotidine (Pepcid IV), fentanyl, filgrastim (Neupogen), fluconazole (Diflucan), gallium nitrate (Ganite), gentamicin, granisetron (Kytril), heparin, hydrocortisone sodium succinate (Solu-Cortef), hydromorphone (Dilaudid), imipenem-cilastatin (Primaxin), linezolid (Zyvox), lorazepam (Ativan), magnesium sulfate, melphalan (Alkeran), meperidine (Demerol), meropenem (Merrem IV), methylprednisolone (Solu-Medrol), metoclopramide (Reglan), metronidazole (Flagyl IV), milrinone (Primacor), morphine, multivitamins (M.V.I.), nafcillin (Nallpen), nalbufine, oxacillin (Bactocill), paclitaxel (Taxol), pemetrexed (Alimta), penicillin G potassium, pentobarbital (Nembutal), potassium chloride (KCl), propofol (Diprivan), ranitidine (Zantac), remifentanil (Ultiva), sodium bicarbonate, sulfamethoxazole/trimethoprim, teniposide (Vumon), theophylline, thiotepa, tobramycin, vancomycin, zidovudine (AZT, Retrovir).
Rate of administration
A single dose must be administered at a constant rate over 1 hour as an infusion. Renal tubular damage will occur with too-rapid rate of injection. Acyclovir crystals will occlude renal tubules. Use of an infusion pump or microdrip (60 gtt/mL) recommended.
Actions
An antiviral agent with activity against herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) and varicella-zoster virus (VZV). Inhibits replication of viral DNA. Widely distributed in tissues and body fluids. Metabolized to a small extent in the liver. Half-life is approximately 2.5 hours. Excreted mainly as unchanged drug in the urine. Crosses the placental and blood-brain barriers. Secreted in breast milk.
Indications and uses
Treatment of initial and recurrent mucosal and cutaneous herpes simplex (HSV-1 and HSV-2) infections in immunosuppressed patients. ■ Severe initial clinical episodes of herpes genitalis in immunocompetent patients. ■ Herpes simplex encephalitis. ■ Neonatal herpes simplex virus infections. ■ Herpes zoster infections (shingles) in immunocompromised patients. ■ Oral acyclovir is used to treat varicella zoster (chickenpox).
Unlabeled uses:
Prevention of HSV reactivation in HSCT, treatment of disseminated HSV or VZV, or empiric treatment of suspected encephalitis in immunocompromised patients with cancer.
Contraindications
Hypersensitivity to acyclovir or valacyclovir (Valtrex). The ganciclovir monograph indicates a contraindication for ganciclovir with acyclovir.
Precautions
Confirm diagnosis of herpes simplex virus (HSV-1 or HSV-2) through laboratory culture. Initiate therapy as quickly as possible after symptoms are identified. ■ For IV use only; avoid IM or SC injection. ■ Use caution in patients with underlying neurologic abnormalities, those with serious renal, hepatic, or electrolyte abnormalities, or significant hypoxia. ■ Use caution in patients receiving interferon or intrathecal methotrexate, or with patients who have had previous neurologic reactions to cytotoxic drugs. ■ Incidence of CNS adverse events may be more common in the elderly or in patients with decreased renal function. ■ Isolates of herpes simplex viruses (HSV-1, HSV-2) and varicella-zoster virus (VZV) with reduced susceptibility to acyclovir have been identified. Consider the possibility of viral resistance to acyclovir in patients who show poor clinical response. ■ Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS) has been reported in immunocompromised patients receiving acyclovir. Deaths have occurred. ■ See Contraindications.
Monitor:
Maintain adequate hydration and urine flow before and during infusion. Encourage fluid intake of 2 to 3 L/day. ■ Monitor CBC, liver function tests, and renal function; abnormal renal function (decreased CrCl can occur), concomitant use of other nephrotoxic drugs, pre-existing renal disease, and dehydration make further renal impairment with acyclovir more likely. ■ Confirm patency of vein; will cause thrombophlebitis. Rotate site of infusion.
Patient education:
Maintain adequate hydration. Virus remains dormant and can still spread to others. ■ Avoid sexual intercourse when visible herpes lesions are present. Use condoms routinely.
Maternal/child:
Category B: use during pregnancy only if benefits outweigh risk to fetus. ■ Breast milk concentrations can be higher than maternal serum concentrations. Discontinue breast-feeding or evaluate very carefully. ■ 10 mg/kg and 20 mg/kg doses in pediatric patients from 3 months to 16 years achieved concentrations similar to those in adults receiving 5 mg/kg to 10 mg/kg. ■ Use with caution in neonates; they have an age-related decrease in clearance and an increase in half-life (3.8 hours).
Elderly:
Effectiveness is similar to younger adults. ■ Plasma concentrations are higher in the elderly compared to younger adults; may be due to age-related changes in renal function. ■ Duration of pain after healing was longer in patients 65 years or older. ■ Incidence of side effects (e.g., CNS adverse events [coma, confusion, hallucinations, somnolence], dizziness, nausea, renal adverse events, vomiting) was increased. ■ See Dose Adjustments and Precautions.
Drug/lab interactions
See Precautions. May cause neurotoxicity (e.g., severe drowsiness and lethargy) with zidovudine. ■ Concurrent use with other nephrotoxic agents (e.g., aminoglycosides [gentamicin, tobramycin], cisplatin) may increase risk of nephrotoxicity, especially in patients with pre-existing renal impairment. ■ Potentiated by probenecid. ■ Synergistic effects with ketoconazole and interferon have been noted. Clinical importance not established. ■ In one case report, a patient stabilized on phenytoin and valproic acid experienced seizures and a reduction in antiepileptic drug serum concentrations when acyclovir was added to the regimen.
Side effects
Acute renal failure, aggressive behavior, agitation, angioedema, ataxia, coma, confusion, delirium, diaphoresis, disseminated intravascular coagulation (DIC), dizziness, dysarthria (difficulty articulating words), elevated transaminase levels, encephalopathy, hallucinations, headache, hematuria, hemolysis, hives, hyperbilirubinemia, hypersensitivity reactions (e.g., anaphylaxis), hypotension, inflammation at injection site, lethargy, nausea, obtundation, phlebitis, rash, seizures, transient increased BUN or SCr levels, tremors, vomiting. Some patients (fewer than 1%) may have abdominal pain, anemia, anorexia, anuria, chest pain, diarrhea, edema, fatigue, fever, hemoglobinemia, hepatitis, hypokalemia, ischemia of digits, jaundice, leukocytosis, light-headedness, myalgia, neutropenia, neutrophilia, paresthesia, psychosis, pulmonary edema with cardiac tamponade, rigors, skin reactions (e.g., erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), thirst, thrombocytosis, thrombocytopenia, visual abnormalities.
Antidote
Notify physician of all side effects. Discontinue drug with onset of CNS side effects. Treatment will be symptomatic and supportive. Adequate hydration is indicated to prevent precipitation of acyclovir in the renal tubules. A 6-hour session of hemodialysis will reduce plasma acyclovir concentration by approximately 60%. Hemodialysis may be indicated in patients with acute renal failure and anuria. Treat anaphylaxis and resuscitate as necessary.
Adenosine
(ah-DEN-oh-seen)
Adenocard, Adenoscan
Antiarrhythmic
Diagnostic agent
pH 4.5 to 7.5
Usual dose
Adenocard: Conversion of acute paroxysmal supraventricular tachycardia (PSVT):
6 mg initially as a rapid bolus by the peripheral IV route. If supraventricular tachycardia not eliminated in 1 to 2 minutes, give 12 mg. May repeat 12-mg dose in 1 to 2 minutes if needed. Do not exceed 12 mg in any single dose. Give undiluted directly into a vein. If given into an IV line, use the port closest to the insertion site and follow with a rapid NS flush to be certain the solution reaches the systemic circulation.
Do not administer a repeat dose to patients who develop a high-level block on one dose of adenosine.
Adenoscan: Noninvasive diagnosis of coronary artery disease with thallium tomography:
140 mcg/kg/min (0.14 mg/kg/min) as a 6-minute continuous peripheral infusion (total dose of 0.84 mg/kg). Injection should be as close to the venous access as possible. Inject thallium at 3 minutes. May be injected directly into adenosine infusion set. Dose should be based on total body weight. There are no data on the safety or efficacy of alternative Adenoscan infusion protocols. Safety and efficacy of Adenoscan administered by the intracoronary route have not been established. ■ See Drug/Lab Interactions.
Pediatric dose
See Maternal/Child.
Adenocard: Conversion of acute paroxysmal supraventricular tachycardia (PSVT) in pediatric patients weighing less than 50 kg:
0.05 to 0.1 mg/kg. May increase dose by 0.05 to 0.1 mg/kg increments every 2 minutes until PSVT is terminated or maximum dose is reached (0.3 mg/kg or 12 mg). AHA guidelines recommend 0.1 mg/kg rapid IV push. Follow each dose with a 5- to 10-mL NS flush. Double to 0.2 mg/kg if a second dose is required. Maximum first dose 6 mg. Maximum second dose and maximum single dose is 12 mg. ■ See comments in Usual Dose.
Pediatric patients weighing more than 50 kg:
Same as Usual Dose.
Dose adjustments
Metabolism of adenosine is independent of hepatic or renal function. No dose adjustment indicated. ■ See Drug/Lab Interactions; alternative therapy (e.g., calcium channel blockers) may be indicated.
Dilution
Solution must be clear; do not use if discolored or particulate matter present. Give undiluted for both indications.
Storage:
Store at CRT 15° to 30° C (59° to 86° F); refrigeration will cause crystallization. If crystals do form, dissolve by warming to room temperature. Discard unused portion.
Compatibility
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer states that thallium-201 is compatible with Adenoscan and may be injected directly into the Adenoscan infusion set.
One source suggests the following compatibilities:
Y-site:
Abciximab (ReoPro).
Rate of administration
Adenocard:
Conversion of acute PSVT:
Must be given as a rapid bolus IV injection over 1 to 2 seconds. Follow each dose with NS flush; see Usual Dose.
Adenoscan:
Pharmacologic stress testing:
See Usual Dose.
Actions
A naturally occurring nucleoside present in all cells of the body. Has many functions. When given as a rapid IV bolus of 6 or 12 mg, adenosine usually has no systemic hemodynamic effects. As a rapid IV bolus, it has antiarrhythmic properties, slowing cardiac conduction (particularly at the AV node), interrupting re-entry pathways through the AV node, and restoring sinus rhythm in patients with PSVT, including PSVT associated with Wolff-Parkinson-White syndrome. When used as a diagnostic aid and given as a continuous infusion, adenosine acts as a vasodilator. Dilates normal coronary vessels, increasing blood flow. Has little effect on stenotic arteries. When administered with thallium-201, helps differentiate between areas of heart supplied by normal blood flow and areas supplied by stenotic coronary arteries. When larger doses are given by infusion, adenosine decreases BP and produces a reflexive increase in HR. Adenocard and Adenoscan have the same molecular structure, same solvent, diluent, and concentration. The difference in their actions is in the rate of administration; however, the FDA has approved Adenocard for converting PSVT and Adenoscan for pharmacologic stress testing. When used for treatment of PSVT, it is effective within 1 minute. When used for diagnostic purposes, maximum effect is reached within 2 to 3 minutes of starting the infusion. Coronary blood flow velocity returns to basal levels within 1 to 2 minutes after the infusion is discontinued. Half-life is estimated to be less than 10 seconds. Adenosine is salvaged immediately by erythrocytes and blood vessel endothelial cells and metabolized for natural uses throughout the body (regulation of coronary and systemic vascular tone, platelet function, lipolysis in fat cells, intracardiac conduction).
Indications and uses
Adenocard:
To convert acute paroxysmal supraventricular tachycardia (PSVT) to normal sinus rhythm; a first-line agent according to AHA. Includes PSVT associated with accessory bypass tracts (Wolff-Parkinson-White syndrome). Does not convert atrial flutter, atrial fibrillation, or ventricular tachycardia to normal sinus rhythm (NSR).
Adenoscan:
Adjunct to thallium-201 myocardial perfusion scintigraphy in patients unable to exercise adequately. (Results are similar to exercise stress testing.)
Contraindications
Known hypersensitivity to adenosine. ■ Sinus node disease, such as symptomatic bradycardia or sick sinus syndrome, and second- or third-degree AV block unless a functioning artificial pacemaker is in place. ■ Known or suspected bronchoconstrictive or bronchospastic lung disease (e.g., asthma).
Precautions
Both preparations:
Emergency resuscitation drugs and equipment must always be available. ■ May produce short-lasting first-, second-, or third-degree heart block or sinus bradycardia. Usually self-limiting due to short half-life. Patients who develop high-level block should not be given additional doses of adenosine. ■ May cause dyspnea, bronchoconstriction, and respiratory compromise. Use with caution in patients with obstructive lung disease not associated with bronchoconstriction (e.g., emphysema, bronchitis). Avoid use in patients with bronchoconstrictive or bronchospastic disease (e.g., asthma); see Contraindications.
Adenocard:
Valsalva maneuver may be used before use of adenosine in PSVTs if clinically appropriate. ■ Transient or prolonged episodes of asystole and ventricular fibrillation have been reported. Deaths have occurred. In most instances, these cases were associated with the concomitant use of digoxin (Lanoxin) and, less frequently, with digoxin and verapamil. ■ Some slowing of ventricular response may occur if atrial flutter or fibrillation is also present.
Adenoscan:
Use with caution in patients with pre-existing first-degree AV block or bundle branch block. ■ Fatal and nonfatal cardiac arrest, sustained ventricular tachycardia (requiring resuscitation), and MI have been reported. Avoid use in patients with S/S of myocardial ischemia (e.g., unstable angina) or cardiovascular instability; may be at increased risk for adverse cardiac events. ■ Can cause significant hypotension. Use with caution in patients with autonomic dysfunction, stenotic valvular heart disease, pericarditis or pericardial effusion, stenotic carotid artery disease with cerebrovascular insufficiency, or uncorrected hypovolemia; may be at increased risk for hypotensive complications. ■ Hemorrhagic and ischemic cerebrovascular accidents have occurred. ■ New onset or recurrence of convulsive seizures has occurred following administration of Adenoscan. Some seizures are prolonged and require emergent anticonvulsive management. Aminophylline may increase the risk of seizures associated with Adenoscan; see Antidote. ■ Hypersensitivity reactions (e.g., chest discomfort, dyspnea, erythema, flushing, throat tightness, and rash) have occurred. ■ Hypertension has been reported; usually resolves spontaneously within several minutes but, in some cases, has lasted for several hours. ■ Atrial fibrillation has been reported. In reported cases, it began 1.5 to 3 minutes into the infusion, lasted for 15 seconds to 6 hours, and spontaneously converted to NSR.
Monitor:
Adenocard: Conversion of acute PSVT:
Must reach systemic circulation; see Usual Dose. ■ ECG monitoring during administration recommended. Monitor BP. At the time of conversion to normal sinus rhythm, PVCs, PACs, atrial fibrillation, sinus bradycardia, sinus tachycardia, skipped beats, and varying degrees of AV nodal block are seen on the ECG in many patients. Usually last only a few seconds and resolve without intervention. ■ Less likely to precipitate hypotension if arrhythmia does not terminate.
Adenoscan: Pharmacologic stress testing:
ECG monitoring during administration recommended. Monitor HR and BP at regular intervals during infusion. ■ Obtain images when infusion complete and redistribution images 3 to 4 hours later. ■ Monitor for S/S of hypersensitivity reactions.
Patient education:
Avoid consumption of any products containing methylxanthines, including caffeinated coffee, tea, or other caffeinated beverages; caffeine-containing drug products; aminophylline; and theophylline before the myocardial perfusion imaging study. ■ Review medical history (e.g., history of seizures or respiratory compromise). ■ Promptly report any potential side effects.
Maternal/child:
Category C: use in pregnancy only if clearly needed. Some references recommend avoiding early pregnancy. ■ Safety for use in pediatric patients not established, but has been used for conversion of PSVT in neonates, infants, children, and adolescents. Safety for use in pharmacologic stress testing in patients under 18 years not established. ■ Interrupt nursing after administration of Adenoscan.
Elderly:
Response similar to that seen in younger patients; however, greater sensitivity of some older patients cannot be ruled out. May have diminished cardiac function, nodal dysfunction, concomitant disease, or drug therapy that may alter hemodynamic function and produce severe bradycardia or AV block.
Drug/lab interactions
Both preparations:
Effects antagonized by methylxanthines (e.g., caffeine, theophylline); larger doses may be required or adenosine may not be effective. ■ Potentiated by dipyridamole (Persantine); smaller doses of adenosine may be indicated. Safety and efficacy of adenosine in the presence of dipyridamole have not been systematically evaluated. ■ Cardiovascular effects increased by nicotine; rapid injection may induce anginal pain. ■ May produce a higher degree of heart block with carbamazepine (Tegretol). ■ Concomitant use with digoxin alone or digoxin in combination with verapamil associated with rare cases of ventricular fibrillation; see Precautions. ■ See Usual Dose. ■ Adenoscan: Before using Adenoscan for pharmacologic stress testing, avoid (or withhold for at least 5 half-lives) adenosine antagonists (e.g., methylxanthines) and/or potentiators (e.g., dipyridamole). ■ Has been used with other cardioactive agents such as beta-adrenergic blockers (e.g., metoprolol [Lopressor], atenolol [Tenormin]), angiotensin-converting enzyme inhibitors (e.g., lisinopril [Prinivil, Zestril], enalapril [Vasotec]), and calcium channel blockers (e.g., diltiazem [Cardizem], verapamil [Calan]) without apparent adverse interactions, but its effectiveness with these agents has not been fully evaluated.
Side effects
Both preparations:
Generally predictable, short lived, and easily tolerated. Most will appear immediately and last less than 1 minute. Atrial fibrillation, bronchospasm, chest pressure or discomfort, dizziness, dyspnea and/or shortness of breath, facial flushing, GI discomfort, headache, hypertension, light-headedness, nausea, numbness, PACs, PVCs, sinus bradycardia, sinus tachycardia, skipped beats, varying degrees of AV nodal block. Less than 1% of patients complain of apprehension; blurred vision; burning; chest pain; head pressure; heavy arms; hyperventilation; hypotension; metallic taste; neck and back pain; palpitations; pressure in groin; seizures; sweating; throat, neck, or jaw discomfort; tight throat; and tingling in arms. Serious side effects may include arrhythmias (persistent), including VT, VF, atrial fibrillation, and torsades de pointes; bronchospasm (severe); myocardial infarction; pulmonary edema; third-degree AV block. Asystole with fatal outcome has been reported.
Post-marketing:
Both preparations:
Seizure activity, including tonic-clonic (grand mal) seizures, and loss of consciousness. Adenoscan: Cardiac failure, cerebrovascular accident (including intracranial hemorrhage), fatal and nonfatal cardiac arrest, hypersensitivity reactions, injection site reactions, myocardial infarction, respiratory arrest, throat tightness, ventricular arrhythmia, vomiting. Adenocard: Atrial fibrillation, bradycardia, prolonged asystole, torsades de pointes, transient increase in blood pressure, VF, VT.
Antidote
Notify physician of any side effect that lasts more than 1 minute. If a side effect persists, decrease rate of infusion (Adenoscan [pharmacologic stress testing]). Discontinue in any patient who develops severe respiratory difficulties or in any patient who develops persistent or symptomatic high-grade AV block or hypotension. Treat side effects symptomatically if indicated. Bradycardia may be refractory to atropine. Short half-life generally precludes overdose problems, but aminophylline 50 to 125 mg as a slow infusion is a competitive antagonist. Methylxanthine use (e.g., aminophylline) is not recommended in patients who experience seizures in association with Adenoscan. Resuscitate as necessary.
Ado-trastuzumab emtansine 
(a-do-tras-TU-zoo-mab em-TAN-seen)
Kadcyla
Monoclonal antibody
Antineoplastic
pH 5
Usual dose
3.6 mg/kg as an infusion every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity. Do not administer doses greater than 3.6 mg/kg. Do not substitute ado-trastuzumab (Kadcyla) for or with trastuzumab.
Dose adjustments
No dose adjustment is indicated in patients with mild or moderate renal impairment. In patients with severe renal impairment (CrCl less than 30 mL/min), no dose adjustments can be recommended because of the limited data available. ■ If a planned dose is delayed or missed, do not wait until the next planned cycle; administer as soon as possible, and adjust the schedule to maintain a 3-week interval between doses. ■ The recommended dose reduction schedule for adverse events and dose modification guidelines for various adverse events are listed in the following charts.
| Recommended Ado-Trastuzumab Dose Reduction Schedule for Adverse Events* | |
| Dose Reduction Schedule | Dose Level to Administer |
| Starting dose | 3.6 mg/kg |
| First dose reduction | 3 mg/kg |
| Second dose reduction | 2.4 mg/kg |
| Requirement for further dose reduction | Discontinue treatment |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


