Chapter 9 Diabetes mellitus
RELEVANT ANATOMY AND PHYSIOLOGY OF PANCREAS
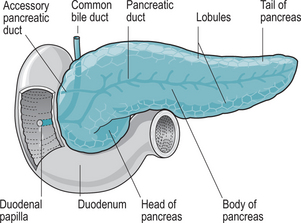
The pancreas is an oblong gland, approximately 12.5 cm long and 2.5 cm thick. It is situated in the epigastric region posterior to the greater curvature of the stomach, and is connected by two ducts to the duodenum (Fig. 9.1). The head is the expanded portion situated near the ‘C’ shaped curve of the duodenum, superior and to the left are the central body and tail.
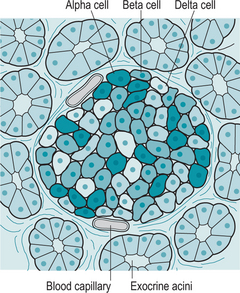
Interspersed within the acini are small clusters of cells called the islets of Langerhans or pancreatic islets, which form the endocrine portion of the pancreas (Fig. 9.2). There are four types of cells within the islets, which secrete the following hormones:
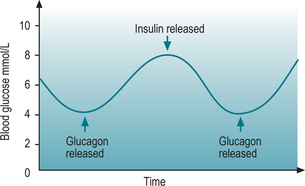
Secretion of glucagon is via a negative feedback system (Fig. 9.3); if the blood glucose level falls below normal, chemical sensors stimulate the alpha cells to secrete glucagon. When blood glucose level rises, the cells are no longer stimulated and production ceases. Exercise and protein-heavy meals also stimulate glucagon secretion. Somatostatin inhibits glucagon secretion.
Emotional stress commonly raises blood glucose levels. Cortisol is a corticosteroid hormone produced by the adrenal cortex and is involved in the response to stress. It increases blood pressure and blood glucose levels. Cortisol has widespread actions which help to restore homeostasis after stress. Cortisol acts as a physiological antagonist to insulin by promoting gluconeogenesis, the breakdown of fats and proteins and the mobilization of amino acids and ketone bodies. This leads to an increase in blood glucose levels and the storage of glycogen in the liver (Marieb & Hoehn 2007).
Regulation of insulin is determined by the glucose level in the blood. Increased blood glucose levels stimulate insulin secretion, while decreased blood glucose levels inhibit it (Fig. 9.3). Increased levels of certain amino acids also stimulate insulin release. Human growth hormone (hGH) and adrenocorticotrophic hormone (ACTH) both raise blood glucose levels, and the rise in blood glucose level stimulates insulin secretion. Somatostatin inhibits the secretion of insulin.
TYPES OF DIABETES MELLITUS
Type 1 diabetes mellitus
Aetiology
The aetiology of type 1 diabetes mellitus is thought to be multifactorial and far from being completely understood. It is considered to have an autoimmune basis and a number of risk factors have been identified, which may be divided into genetic and environmental. These risk factors include links to chromosomes and several chromosomal regions have been implicated. The most significant gene loci defining the risk of type 1 diabetes are to be found within leucocyte antigen (HLA) gene region (BMA 2004).
There has been a rapidly rising incidence in migrant populations suggesting environmental links, for example viral infections. It has been observed that IgM antibodies to Coxsackie B virus are found in 25–35% of newly diagnosed diabetics (Kanno et al 2006). An increased incidence of type 1 diabetes has been noted in individuals with congenital rubella, and cytomegalovirus DNA has also been found in 22% of newly diagnosed diabetics (Jaeckel et al 2002).
It has also been suggested that those with diabetes were breast-fed for a significantly shorter time than non-diabetic infants, and that the incidence of diabetes can be modified by the exclusion of cow’s milk, suggesting that early exposure to cow’s milk may be an important factor in the aetiology of type 1 diabetes (BMA 2004). The condition varies considerably in incidence between cultures with the 10 countries with the highest proportion of sufferers being India, China, USA, Indonesia, Japan, Pakistan, Russia, Brazil, Italy and Bangladesh (WHO 2006).
Incidence
The incidence of diabetes mellitus appears to be on the increase, and a likely factor related to this increase is obesity and lifestyle in western culture. The prevalence of diabetes for all age-groups worldwide was estimated to be 2.8% in 2000 and is estimated to rise to 4.4% by 2030 (Wild et al 2004). The total number of people with diabetes is projected to rise from 171 million in 2000 to 366 million in 2030.
The costs to people’s quality of life, the economy, society and the NHS, are already evident. Diabetes can lead to serious complications, such as heart disease, blindness, kidney failure and stroke and nerve damage, leading to amputation (Diabetes UK 2006).
There are several reasons why the prevalence of diabetes is increasing: the greater life expectancy means that there is an increasingly ageing population. Also, the UK has a rapidly growing obesity problem, and the risk of developing type 2 diabetes increases by up to 10 times in people with a body mass index (BMI) >30. In people with an African-Caribbean or Asian background, the prevalence is approximately five times higher than that of the indigenous population (BMA 2004).
Signs and symptoms
Classically, the three ‘polys’ are described:
Polyuria – excessive micturition
Polyphagia – voracious appetite.
Pathophysiology
Uncontrolled and untreated, these features will lead to a life-threatening condition known as diabetic ketoacidosis (McIntyre & Strachan 2000).
Management
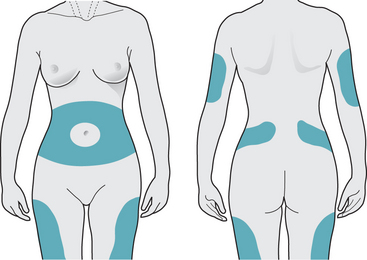
Giving insulin injections is usually a concern, and must be demonstrated and practised carefully by the individual until they feel confident. Alcohol swabbing is contraindicated, as it causes the skin to toughen, making injections more difficult. Needle length, angle of injection, as well as suitable sites for injection must also be addressed (Box 9.1, Fig. 9.4). Various devices are available to deliver insulin to the individual, including insulin pens, which are loaded with a single use cartridge containing the required dose.
Box 9.1 Administration of insulin
Insulin is administered by subcutaneous injection. There are three main areas for injection – abdomen, buttocks and thighs (Fig. 9.4). The upper arm is the least preferred site as it has little subcutaneous tissue. Overuse of one site can lead to fat hypertrophy and loss of sensitivity. If insulin is injected into fatty tissue, this will result in slower and more erratic absorption of insulin, resulting in variable and unstable blood glucose readings. It is advisable to use a different site each day and by rotating sites, this ensures the insulin will act quickly and effectively.
Glucose tests are usually carried out four times daily before meals and at bedtime and should aim to keep blood glucose levels between 4–7 mmol/L. The advantage of daily blood glucose monitoring is that it provides day-to-day control and greater patient involvement in managing their diabetes.
Urinalysis is not routinely carried out, as it is unhelpful for all those using insulin. It does not diagnose low blood glucose levels nor can it be used to identify a hypoglycaemic event. Furthermore, it is considered that urinalysis provides neither an accurate nor a precise measurement of high or low blood glucose levels (Diabetes UK 2006).
The most important issues are that the individual must be supported in this very difficult time and that she has access to specialist diabetic care resources and self-help groups. ‘Buddy’ support groups where an experienced diabetic teams up with a newly diagnosed individual are useful but must not replace professional support (Scottish Diabetes Framework 2006).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree