- Structure and physiological function of troponin, CK(MB) and BNP
- Blood collection for troponin, CK(MB) and BNP testing
- Myocardial infarction/acute coronary syndrome (ACS)
- Troponin and CK(MB) testing in assessment of patients with chest pain
- Heart failure
- BNP testing in assessment of patients with suspected heart failure
Chest pain and breathlessness are two common reasons for adults to seek medical help either in primary care or at the hospital emergency department. This chapter is concerned with how laboratory testing can help in the diagnosis of patients presenting with either of these two symptoms. The three tests for consideration here are: the serum or plasma concentration of cardiac troponins (cTnT and cTnI); the serum or plasma concentration of creatine kinase CK(MB); and the plasma concentration of brain natriuretic peptide (BNP). Although functionally and structurally unrelated all three substances are normally present in cardiac tissue cells and released to blood in abnormal amounts if those cells are damaged or diseased: they are thus blood markers of heart (cardiac) disease and referred to collectively as cardiac markers.
The principle use of the first two tests, troponins and CK(MB) is to help identify those patients whose chest pain is due to myocardial infarction, the acute and life threatening manifestation of coronary heart disease (CHD) commonly known as a ‘heart attack’. Every year in the UK around 125 000 people suffer myocardial infarction1. The damage to the heart may be sufficient to cause immediate lethal arrhythmia, cardiac arrest and sudden death; around 20% die before medical help arrives. For the rest early diagnosis and treatment is usually life-saving; around 90% of those who get to hospital alive now survive myocardial infarction.
The principle use of the third test, serum BNP, is to help identify those patients who are suffering heart failure, the most frequent early symptom of which is breathlessness after only minimal exertion. This is a common, progressively debilitating chronic disease that can result from the damage caused to the heart during myocardial infarction; chronic hypertension is another major cause. Heart failure affects an estimated 900 000 in the UK2. This is a disease of advancing years and the vast majority of those affected are aged more than 65 years at the time of diagnosis. Prevalence is highest in the very elderly (>84 years); around one in ten in this age group have heart failure3.
Normal physiology
Troponin
There are three main muscle types in the human body: smooth muscle, present in the wall of those hollow organs whose function depends on muscle wall contraction (gastrointestinal tract, uterus, blood vessels etc.); skeletal muscle; and cardiac muscle (the myocardium) which makes up the bulk of the heart wall.
Troponin (Tn) is a protein constituent of cardiac and skeletal muscle cells where it functions as a structural component of the contractile assembly (myofibrils) that enables muscle contraction. It is composed of three sub-units: troponin C (TnC), troponin I (TnI) and troponin T(TnT). The whole troponin complex is located on the actin filament of the myofibril. The interaction between actin and myosin filaments that facilitates muscle contraction is initiated by calcium ions binding to troponin C. Tnl binding of actin inhibits contraction. By these two opposing effects, one initiating contraction of myofibrils the other inhibiting the process, troponin plays a major role in regulating contraction of both skeletal and cardiac muscle.
There are tissue specific isoforms of troponins C, I and T. This means that it is possible to distinguish cardiac muscle troponin (cTn) from skeletal muscle troponin. Normally all the troponin in the body is contained within skeletal and cardiac muscle cells; it is virtually undetectable in blood. However if muscle cells are damaged, their contents, including troponin, are released to the bloodstream and plasma concentration rises. If only cardiac muscle is damaged, only troponin composed of the cardiac isoforms of troponin sub-units C, I and T (i.e. cTnC, cTnI, and cTnT) will appear in blood. There are two troponin tests currently used for assessment of patients with chest pain. Some laboratories measure serum or plasma concentration of cTnT; others measure serum or plasma concentration of cTnI. Of many potential candidates, the troponins cTnT and cTnI have emerged as the cardiac markers of choice for diagnosis and exclusion of myocardial infarction because of their superior specificity and sensitivity for cardiac muscle (myocardial) damage.
Creatine kinase (MB) CK(MB)
Creatine kinase (alternative name, creatine phosphokinase CPK) is an enzyme that catalyses the transfer of phosphate from creatine phosphate to adenosine diphosphate. The products of the reaction are creatine and the energy rich compound, adenosine triphosphate (ATP).

CK is present in many types of tissue cells, but three sorts of tissue contain most of the body’s CK. They are: cardiac muscle (myocardium), skeletal muscle and the brain.
CK is composed of two protein subunits, M and B, allowing three functionally identical, but structurally different, isoenzymes: CK(MM), CK(BB) and CK(MB). CK isoenzymes are tissue specific. Most of the CK(BB) is found in the brain; most of the CK(MM) is in skeletal muscle and most of the CK(MB) is in cardiac muscle cells. Thus CK(MB) is a fraction of total CK which is confined to the cells of which heart muscle are composed. Normally blood plasma contains very little CK(MB) but following damage to heart muscle, blood plasma concentration rises. CK(MB) is considered the best alternative cardiac marker, if troponin is not available.
Brain natriuretic peptide (BNP)
Peptide is the generic name for all substances that comprise a simple chain of a small number of amino acids (usually less than 50). Brain natriuretic peptide was first isolated from the brain of pigs, hence the name, but in humans most is actually synthesised in the heart, specifically in the muscle cells (myocytes) that comprise the muscular wall (myocardium) of the two ventricles of the heart. A closely related peptide, atrial natriuretic peptide (ANP) is synthesised in the myocytes that comprise the wall (myocardium) of the two atria of the heart. All natriuretic peptides (there are two other types, not synthesised in the heart) are hormones that are involved in the regulation of the amount of sodium and water in blood and thereby blood volume, blood pressure and ultimately cardiac function. The mode of action and physiological effect of these hormones is complex but an important aspect is that via action on the renin-angiotensin system, they promote the excretion of sodium in urine (natriuresis) and it is this action that is reflected in their collective name.
The physiological impetus for BNP synthesis and secretion to blood is ventricular stretching and distension that occurs when the heart is working harder than normal (e.g. during vigorous exercise). The ventricular myocytes do not actually secrete BNP in response to ventricular stretching but a larger peptide (a pro-hormone) called proBNP, which is composed of 108 amino acids. This is split during secretion, and the derived two substances are both present in blood. The first is the physiologically active hormone BNP, composed of 32 amino acids and the second is a physiologically inactive peptide called N-terminal pro-brain natriuretic peptide (NTproBNP) comprising 76 amino acids. Some laboratories measure BNP concentration and some measure NTproBNP concentrations. The two assays are equally valid for clinical purposes, but because BNP and NTproBNP are metabolised at different rates, each assay has its own, quite different reference (normal) range.
Laboratory measurement of troponins (cTnT cTnI) and CK(MB)
Patient preparation
No particular patient preparation is necessary.
Timing of blood collection
Most hospitals have a protocol for timing of blood sampling for troponin and CK(MB) among patients presenting with chest pain. Commonly blood is sampled on admission and again 6–12 hours later. Further testing at 24 hours may be necessary. Interpretation of test results depends crucially on knowing when the blood was sampled in relation to the time of onset of symptoms of chest pain. For these reasons it is important to record on the accompanying request card both the time blood is sampled and the time of onset of symptoms (if known) or time of admission.
Amount and type of sample
Around 5 ml of blood is sufficient for cardiac markers. The assays are performed on either plasma or serum. If local policy is to use plasma then blood must be collected into a tube containing the anticoagulant lithium heparin. If local policy is to use serum then blood must be collected into a plain glass tube, without any additive. Haemolysed samples are unsuitable for troponin testing – repeat blood sampling is necessary if haemolysis is present.
Interpretation of results
Reference ranges
It would be inappropriate to provide definitive reference ranges for these two tests because of variable and continually evolving laboratory methodology. An important aspect of this, so far as troponin is concerned, is development of assays that are increasingly sensitive in detecting troponin. Interpretation of patient results should always be made using reference ranges provided by the laboratory that performed the test(s). Suffice to say there is normally very little (often undetectable quantities) of any of these cardiac markers in blood.
The now recommended unit of measurement for Tnl and T is nanograms/litre (ng/L) but some laboratories continue to use other units (ng/ml or mg/L). To convert results expressed as either ng/ml or mg/L to the recommended ng/L, simply multiply the result by 1000.
Causes of raised serum or plasma concentration of cTnT, cTnI and CK(MB)
Cardiac muscle cell death (myocardial necrosis) is the only cause for an increase in serum or plasma concentration of cTnT and cTnI. It is also the principle but not the sole cause for an increase in plasma or serum concentration of CK(MB). The most common cause of myocardial necrosis is myocardial infarction.
Myocardial infarction/coronary heart disease
All cells require a continuous supply of oxygen rich blood for survival. Ischaemia is the term used to describe deficient blood supply to an area of tissue, and infarction is the term for the death of tissue that results if ischaemia is sufficiently severe or prolonged. Myocardial infarction is thus the death of an area of heart muscle tissue due to ischaemia. Almost invariably in cases of myocardial infarction, ischaemia is the result of CHD.
The atherosclerotic plaque is the pathological lesion that defines CHD. This is a focal accumulation of lipid and cellular material beneath a fibrous cap on the internal surface of a coronary artery. During a long sub-clinical period of many years the plaque may grow to the point where it reduces blood flow sufficiently to cause symptoms of reduced oxygen delivery to an area of heart muscle. The main symptom is ischaemic chest pain, which is often experienced as discomfort rather than as a sharp or stabbing pain. Tightness, pressure, constriction and strangling are common descriptors of ischaemic chest pain. It is usually diffusely located across the chest and may radiate to neck, throat, jaw, shoulders or arm.
The least severe and most common manifestation of CHD is the ischaemic chest pain associated with stable angina. A patient with stable angina experiences chest pain or discomfort only during periods of increased oxygen demand, for example, during exertion or other stress (e.g. emotional stress) that causes heart rate to increase. Symptoms disappear as the heart’s demand for oxygen is reduced by rest. The reduced oxygen delivery to heart muscle cells that causes ischaemic pain in those with stable angina is not sufficient to cause cell death. There is therefore no increase in the serum or plasma concentration of either of the two cardiac markers among patients whose chest pain is the result of stable angina.
The first manifestation of CHD may not be stable angina but the more serious acute coronary syndrome (ACS). This is not a single entity but a spectrum of disease of increasing severity that includes myocardial infarction. The pathological feature that defines ACS is plaque instability. For reasons that remain poorly understood, the fibrous cap that protects the underlying lipid and cellular contents of an atherosclerotic plaque from the blood flowing through an affected artery, may be thin, fragile and prone to disruption (rupture). It is the patients whose plaques have these vulnerable characteristics that are most at risk of the life threatening consequences of CHD. Plaque rupture exposes the platelets in blood to the pro-coagulant environment of the plaque contents. A single thrombus may form at the site of the exposed plaque, partially or totally occluding blood flow. Alternatively debris from the plaque along with fragments of thrombi may embolise to smaller vessels where they may occlude blood flow at a site remote from the plaque. It is in the context of these variable effects of plaque disruption that ACS is evident.
The mildest clinical presentation of ACS is unstable angina. This is the same ischaemic chest pain or discomfort as that experienced by patients suffering stable angina. However in the case of unstable angina, symptoms are experienced at rest or on minimal exertion, and are generally of longer duration and greater intensity. A definitive feature of unstable angina and one that distinguishes it from the more serious presentation of ACS, myocardial infarction, is the absence of myocardial necrosis. As with stable angina, the ischaemia associated with unstable angina is not sufficient to cause cell death, so that cardiac markers remain within normal limits. However unstable angina is clinical evidence of an unstable plaque and therefore high risk of myocardial infarction in the immediate future or later. Around 15% of patients with unstable angina suffer myocardial infarction during the seven days following diagnosis.
Myocardial infarction and cardiac markers
If the ischaema induced by thrombus or other occlusion of an artery is severe and prolonged, the myocardial cells that are supplied by the vessel simply die. This is myocardial infarction. The volume of myocardium affected varies greatly from <1 to >25 g depending on the site of occlusion, and this is reflected in the variable electrocardiographic (ECG) changes and mortality associated with myocardial infarction. In all cases there is an increase in the plasma concentration of cardiac markers, indeed an increase in the plasma concentration of troponin (either cTnT or cTnI) or CK(MB) is an essential criteria for diagnosis of myocardial infarction 4 (Table 9.1). The magnitude of the increase reflects the amount of tissue destroyed and therefore severity of the infarct.
Crushing ischaemic chest pain, usually lasting no less than 20 minutes marks the onset of severe myocardial infarction in the majority of cases. Additional symptoms include breathlessness, light-headedness, sweating, nausea and vomiting. Extensive tissue ischaemia is reflected in the early characteristic electrocardiograph (ECG) change (ST elevation) that gives this severe presentation its name: ST elevation myocardial infarction (STEMI). Myocardial necrosis is not immediate and begins only after a finite period (≈10–15 minutes) of ischaemia. Necrosis of all the tissue at risk occurs gradually over the following four to six hours or longer, providing a window of therapeutic opportunity to limit the damage with thrombolytic/reperfusion therapy that restores blood flow.
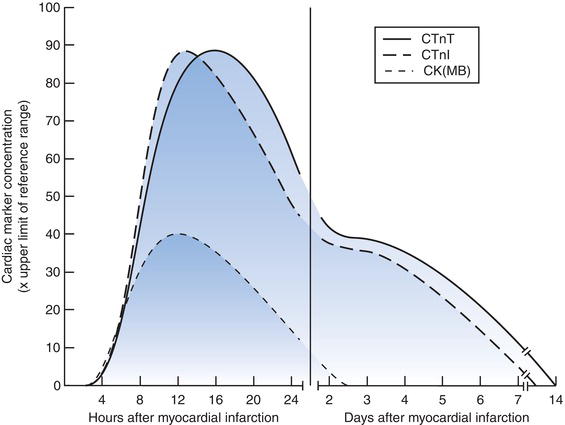
The rise in plasma concentration of cTnI, cTnT and CK(MB) following myocardial infarction is a transitory phenomenon, related to the time of infarction and typically begins between three and six hours after onset of symptoms, although may be earlier in particular cases (Figure 9.1). Peak levels occur at about 12–24 hours after onset of symptoms. CK(MB) concentration returns to normal within two or thress days but both cTnI and cTnT remain raised for much longer, up to two weeks in some cases.
Table 9.1 Definition of myocardial infarction4.
| The following criteria satisfy the diagnosis of acute, evolving or recent MI: |
Detection of rise and/or fall of a blood cardiac marker (preferably troponin), with at least one value above the 99th percentile of the upper reference limit. This evidence of myocardial necrosis must be accompanied by evidence of ischaemia; that is, at least one of the following:
|
Figure 9.1 Typical time related change in cardiac marker concentration following myocardial infarction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


